Structures of the σ2 receptor enable docking for bioactive ligand discovery
- PMID: 34880501
- PMCID: PMC8867396
- DOI: 10.1038/s41586-021-04175-x
Structures of the σ2 receptor enable docking for bioactive ligand discovery
Abstract
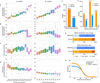
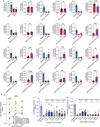
The σ2 receptor has attracted intense interest in cancer imaging1, psychiatric disease2, neuropathic pain3-5 and other areas of biology6,7. Here we determined the crystal structure of this receptor in complex with the clinical candidate roluperidone2 and the tool compound PB288. These structures templated a large-scale docking screen of 490 million virtual molecules, of which 484 compounds were synthesized and tested. We identified 127 new chemotypes with affinities superior to 1 μM, 31 of which had affinities superior to 50 nM. The hit rate fell smoothly and monotonically with docking score. We optimized three hits for potency and selectivity, and achieved affinities that ranged from 3 to 48 nM, with up to 250-fold selectivity versus the σ1 receptor. Crystal structures of two ligands bound to the σ2 receptor confirmed the docked poses. To investigate the contribution of the σ2 receptor in pain, two potent σ2-selective ligands and one potent σ1/σ2 non-selective ligand were tested for efficacy in a mouse model of neuropathic pain. All three ligands showed time-dependent decreases in mechanical hypersensitivity in the spared nerve injury model9, suggesting that the σ2 receptor has a role in nociception. This study illustrates the opportunities for rapid discovery of in vivo probes through structure-based screens of ultra large libraries, enabling study of underexplored areas of biology.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures








References
-
- Waarde A van et al. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochimica Et Biophysica Acta Bba - Biomembr 1848, 2703–2714 (2015). - PubMed
-
- Harvey PD et al. Effects of Roluperidone (MIN-101) on two dimensions of the negative symptoms factor score: Reduced emotional experience and reduced emotional expression. Schizophr Res 215, 352–356 (2020). - PubMed
-
- Intagliata S et al. Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]imidazol-2(3H)-one (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo. Aaps J 22, 94 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

