PrP C as a Transducer of Physiological and Pathological Signals
- PMID: 34880726
- PMCID: PMC8648500
- DOI: 10.3389/fnmol.2021.762918
PrP C as a Transducer of Physiological and Pathological Signals
Abstract
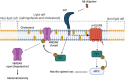
After the discovery of prion phenomenon, the physiological role of the cellular prion protein (PrP C ) remained elusive. In the past decades, molecular and cellular analysis has shed some light regarding interactions and functions of PrP C in health and disease. PrP C , which is located mainly at the plasma membrane of neuronal cells attached by a glycosylphosphatidylinositol (GPI) anchor, can act as a receptor or transducer from external signaling. Although the precise role of PrP C remains elusive, a variety of functions have been proposed for this protein, namely, neuronal excitability and viability. Although many issues must be solved to clearly define the role of PrP C , its connection to the central nervous system (CNS) and to several misfolding-associated diseases makes PrP C an interesting pharmacological target. In a physiological context, several reports have proposed that PrP C modulates synaptic transmission, interacting with various proteins, namely, ion pumps, channels, and metabotropic receptors. PrP C has also been implicated in the pathophysiological cell signaling induced by β-amyloid peptide that leads to synaptic dysfunction in the context of Alzheimer's disease (AD), as a mediator of Aβ-induced cell toxicity. Additionally, it has been implicated in other proteinopathies as well. In this review, we aimed to analyze the role of PrP C as a transducer of physiological and pathological signaling.
Keywords: Alzheimer’s disease; Aβ; PrP; PrPC in CNS; PrPC role; PrPC signaling.
Copyright © 2021 Panes, Saavedra, Pineda, Escobar, Cuevas, Moraga-Cid, Fuentealba, Rivas, Rezaei and Muñoz-Montesino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Avila J., Lucas J. J., Perez M., Hernandez F. (2004). Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 84 361–384. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

