Multi-Omics Comparison of the Spontaneous Diabetes Mellitus and Diet-Induced Prediabetic Macaque Models
- PMID: 34880765
- PMCID: PMC8645867
- DOI: 10.3389/fphar.2021.784231
Multi-Omics Comparison of the Spontaneous Diabetes Mellitus and Diet-Induced Prediabetic Macaque Models
Abstract
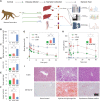
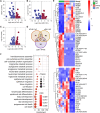
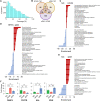
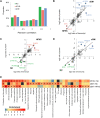
The prevalence of diabetes mellitus has been increasing for decades worldwide. To develop safe and potent therapeutics, animal models contribute a lot to the studies of the mechanisms underlying its pathogenesis. Dietary induction using is a well-accepted protocol in generating insulin resistance and diabetes models. In the present study, we reported the multi-omics profiling of the liver and sera from both peripheral blood and hepatic portal vein blood from Macaca fascicularis that spontaneously developed Type-2 diabetes mellitus with a chow diet (sDM). The other two groups of the monkeys fed with chow diet and high-fat high-sugar (HFHS) diet, respectively, were included for comparison. Analyses of various omics datasets revealed the alterations of high consistency. Between the sDM and HFHS monkeys, both the similar and unique alterations in the lipid metabolism have been demonstrated from metabolomic, transcriptomic, and proteomic data repeatedly. The comparison of the proteome and transcriptome confirmed the involvement of fatty acid binding protein 4 (FABP4) in the diet-induced pathogenesis of diabetes in macaques. Furthermore, the commonly changed genes between spontaneous diabetes and HFHS diet-induced prediabetes suggested that the alterations in the intra- and extracellular structural proteins and cell migration in the liver might mediate the HFHS diet induction of diabetes mellitus.
Keywords: cynomolgus monkey (Macaca fascicularis); metabolomics; non-human primates; proteomics; spontaneous diabetes mellitus; transcriptomics.
Copyright © 2021 Yang, Yang, Tan, Wong, Yang, Zhou, Cai and Lin.
Conflict of interest statement
CWW was employed by the Guangzhou Huazhen Biosciences Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Supplementation of papaya leaf juice has beneficial effects on glucose homeostasis in high fat/high sugar-induced obese and prediabetic adult mice.BMC Complement Med Ther. 2024 Jan 3;24(1):18. doi: 10.1186/s12906-023-04320-1. BMC Complement Med Ther. 2024. PMID: 38172797 Free PMC article.
-
Crosstalk of hepatocyte nuclear factor 4a and glucocorticoid receptor in the regulation of lipid metabolism in mice fed a high-fat-high-sugar diet.Lipids Health Dis. 2022 May 25;21(1):46. doi: 10.1186/s12944-022-01654-6. Lipids Health Dis. 2022. PMID: 35614477 Free PMC article.
-
Psychosocial stress increases risk for type 2 diabetes in female cynomolgus macaques consuming a western diet.Psychoneuroendocrinology. 2022 May;139:105706. doi: 10.1016/j.psyneuen.2022.105706. Epub 2022 Feb 26. Psychoneuroendocrinology. 2022. PMID: 35259592 Free PMC article.
-
Saskatoon berry powder reduces hepatic steatosis and insulin resistance in high fat-high sucrose diet-induced obese mice.J Nutr Biochem. 2021 Sep;95:108778. doi: 10.1016/j.jnutbio.2021.108778. Epub 2021 May 15. J Nutr Biochem. 2021. PMID: 34004342
-
Dietary lipids influence insulin action.Ann N Y Acad Sci. 1993 Jun 14;683:151-63. doi: 10.1111/j.1749-6632.1993.tb35701.x. Ann N Y Acad Sci. 1993. PMID: 8352437 Review.
Cited by
-
Bile acid metabolism in type 2 diabetes mellitus.Nat Rev Endocrinol. 2025 Apr;21(4):203-213. doi: 10.1038/s41574-024-01067-8. Epub 2025 Jan 6. Nat Rev Endocrinol. 2025. PMID: 39757322 Review.
-
Illuminating diabetes via multi-omics: Unraveling disease mechanisms and advancing personalized therapy.World J Diabetes. 2025 Jul 15;16(7):106218. doi: 10.4239/wjd.v16.i7.106218. World J Diabetes. 2025. PMID: 40697608 Free PMC article. Review.
-
Metabolomics reveals soluble epoxide hydrolase as a therapeutic target for high-sucrose diet-mediated gut barrier dysfunction.Proc Natl Acad Sci U S A. 2024 Nov 26;121(48):e2409841121. doi: 10.1073/pnas.2409841121. Epub 2024 Nov 18. Proc Natl Acad Sci U S A. 2024. PMID: 39556751 Free PMC article.
-
Multi-omics analysis reveals changes in tryptophan and cholesterol metabolism before and after sexual maturation in captive macaques.BMC Genomics. 2023 Jun 7;24(1):308. doi: 10.1186/s12864-023-09404-3. BMC Genomics. 2023. PMID: 37286946 Free PMC article.
-
Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome and irritable bowel syndrome.Nat Commun. 2023 Aug 17;14(1):4986. doi: 10.1038/s41467-023-40552-y. Nat Commun. 2023. PMID: 37591886 Free PMC article.
References
-
- American Diabetes A ssociation (2018). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 41 (1), S13–S27. 10.2337/dc18-S002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

