Activation of α7nAChR Protects Against Gastric Inflammation and Dysmotility in Parkinson's Disease Rats
- PMID: 34880768
- PMCID: PMC8646045
- DOI: 10.3389/fphar.2021.793374
Activation of α7nAChR Protects Against Gastric Inflammation and Dysmotility in Parkinson's Disease Rats
Abstract
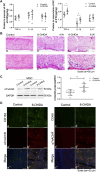
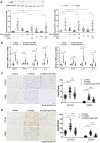
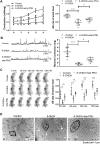
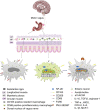
The cholinergic anti-inflammatory pathway (CAIP) has been proposed to regulate gastrointestinal inflammation via acetylcholine released from the vagus nerve activating α7 nicotinic receptor (α7nAChR) on macrophages. Parkinson's disease (PD) patients and PD rats with substantia nigra (SN) lesions exhibit gastroparesis and a decayed vagal pathway. To investigate whether activating α7nAChR could ameliorate inflammation and gastric dysmotility in PD rats, ELISA, western blot analysis, and real-time PCR were used to detect gastric inflammation. In vitro and in vivo gastric motility was investigated. Proinflammatory mediator levels and macrophage numbers were increased in the gastric muscularis of PD rats. α7nAChR was located on the gastric muscular macrophages of PD rats. The α7nAChR agonists PNU-282987 and GTS-21 decreased nuclear factor κB (NF-κB) activation and monocyte chemotactic protein-1 mRNA expression in the ex vivo gastric muscularis of PD rats, and these effects were abolished by an α7nAChR antagonist. After treatment with PNU-282987 in vivo, the PD rats showed decreased NF-κB activation, inflammatory mediator production, and contractile protein expression and improved gastric motility. The present study reveals that α7nAChR is involved in the development of gastroparesis in PD rats and provides novel insight for the treatment of gastric dysmotility in PD patients.
Keywords: 6-hydroxydopamine; Parkinson’s disease; gastric inflammation; macrophage; α7nAChR.
Copyright © 2021 Zhou, Zheng, Zhang, Wang, Yao, Xiu, Liu, Zhang, Feng and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Baladi M. G., Nielsen S. M., McIntosh J. M., Hanson G. R., Fleckenstein A. E. (2016). Prior Nicotine Self-Administration Attenuates Subsequent Dopaminergic Deficits of Methamphetamine in Rats: Role of Nicotinic Acetylcholine Receptors. Behav. Pharmacol. 27 (5), 422–430. 10.1097/FBP.0000000000000215 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

