Physiologically Based Modeling of the Effect of Physiological and Anthropometric Variability on Indocyanine Green Based Liver Function Tests
- PMID: 34880776
- PMCID: PMC8646094
- DOI: 10.3389/fphys.2021.757293
Physiologically Based Modeling of the Effect of Physiological and Anthropometric Variability on Indocyanine Green Based Liver Function Tests
Abstract
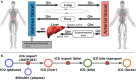
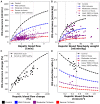
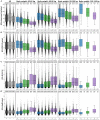
Accurate evaluation of liver function is a central task in hepatology. Dynamic liver function tests (DLFT) based on the time-dependent elimination of a test substance provide an important tool for such a functional assessment. These tests are used in the diagnosis and monitoring of liver disease as well as in the planning of hepatobiliary surgery. A key challenge in the evaluation of liver function with DLFTs is the large inter-individual variability. Indocyanine green (ICG) is a widely applied test compound used for the evaluation of liver function. After an intravenous administration, pharmacokinetic (PK) parameters are calculated from the plasma disappearance curve of ICG which provide an estimate of liver function. The hepatic elimination of ICG is affected by physiological factors such as hepatic blood flow or binding of ICG to plasma proteins, anthropometric factors such as body weight, age, and sex, or the protein amount of the organic anion-transporting polypeptide 1B3 (OATP1B3) mediating the hepatic uptake of ICG. Being able to account for and better understand these various sources of inter-individual variability would allow to improve the power of ICG based DLFTs and move toward an individualized evaluation of liver function. Within this work we systematically analyzed the effect of various factors on ICG elimination by the means of computational modeling. For the analysis, a recently developed and validated physiologically based pharmacokinetics (PBPK) model of ICG distribution and hepatic elimination was utilized. Key results are (i) a systematic analysis of the variability in ICG elimination due to hepatic blood flow, cardiac output, OATP1B3 abundance, liver volume, body weight and plasma bilirubin level; (ii) the evaluation of the inter-individual variability in ICG elimination via a large in silico cohort of n = 100,000 subjects based on the NHANES cohort with special focus on stratification by age, sex, and body weight; (iii) the evaluation of the effect of various degrees of cirrhosis on variability in ICG elimination. The presented results are an important step toward individualizing liver function tests by elucidating the effects of confounding physiological and anthropometric parameters in the evaluation of liver function via ICG.
Keywords: ICG; PBPK; computational model; indocyanine green; liver cirrhosis; liver function; mathematical model; pharmacokinetics.
Copyright © 2021 Köller, Grzegorzewski and König.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Prediction of Survival After Partial Hepatectomy Using a Physiologically Based Pharmacokinetic Model of Indocyanine Green Liver Function Tests.Front Physiol. 2021 Nov 22;12:730418. doi: 10.3389/fphys.2021.730418. eCollection 2021. Front Physiol. 2021. PMID: 34880771 Free PMC article.
-
The Impact of a Heterozygous SLCO1B3 Null Variant on the Indocyanine Green Retention Test.J Pharm Sci. 2020 Oct;109(10):3206-3209. doi: 10.1016/j.xphs.2020.06.020. Epub 2020 Jun 25. J Pharm Sci. 2020. PMID: 32593714
-
Use of Indocyanine Green (ICG), a Medical Near Infrared Dye, for Enhanced Fluorescent Imaging-Comparison of Organic Anion Transporting Polypeptide 1B3 (OATP1B3) and Sodium-Taurocholate Cotransporting Polypeptide (NTCP) Reporter Genes.Molecules. 2019 Jun 21;24(12):2295. doi: 10.3390/molecules24122295. Molecules. 2019. PMID: 31234288 Free PMC article.
-
Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients.Acta Anaesthesiol Scand. 2014 Nov;58(10):1214-9. doi: 10.1111/aas.12406. Acta Anaesthesiol Scand. 2014. PMID: 25307706 Review.
-
Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients.J Clin Monit Comput. 2018 Oct;32(5):787-796. doi: 10.1007/s10877-017-0073-4. Epub 2017 Oct 16. J Clin Monit Comput. 2018. PMID: 29039062 Review.
Cited by
-
Validity and Repeatability Characteristics of a Non-Invasive, Infrared-Based Method Estimating Plasma Indocyanine Green Decay in Healthy Dogs.Animals (Basel). 2023 Nov 9;13(22):3455. doi: 10.3390/ani13223455. Animals (Basel). 2023. PMID: 38003073 Free PMC article.
-
libRoadRunner 2.0: a high performance SBML simulation and analysis library.Bioinformatics. 2023 Jan 1;39(1):btac770. doi: 10.1093/bioinformatics/btac770. Bioinformatics. 2023. PMID: 36478036 Free PMC article.
-
Hepatectomy-Induced Alterations in Hepatic Perfusion and Function - Toward Multi-Scale Computational Modeling for a Better Prediction of Post-hepatectomy Liver Function.Front Physiol. 2021 Nov 18;12:733868. doi: 10.3389/fphys.2021.733868. eCollection 2021. Front Physiol. 2021. PMID: 34867441 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

