Cross-dehydrogenative N-N couplings
- PMID: 34880984
- PMCID: PMC8580018
- DOI: 10.1039/d1sc03851f
Cross-dehydrogenative N-N couplings
Abstract
The relatively high electronegativity of nitrogen makes N-N bond forming cross-coupling reactions particularly difficult, especially in an intermolecular fashion. The challenge increases even further when considering the case of dehydrogenative N-N coupling reactions, which are advantageous in terms of step and atom economy, but introduce the problem of the oxidant in order to become thermodynamically feasible. Indeed, the oxidizing system must be designed to activate the target N-H bonds, while at the same time avoid undesired N-N homocoupling as well as C-N and C-C coupled side products. Thus, preciously few intermolecular hetero N-N cross-dehydrogenative couplings exist, in spite of the central importance of N-N bonds in organic chemistry. This review aims at analyzing these few rare cases and provides a perspective for future developments.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




















References
-
-
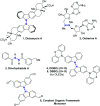
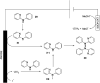
Selected intramolecular N–N bond forming coupling reactions:
- Kotali A. Harris P. A. J. Heterocyclic Chem. 1996;33:605. doi: 10.1002/jhet.5570330312. - DOI
- Sajiki H. Hattori K. Sako M. Hirota K. Synlett. 1997:1409.
- Kurth M. J. Olmstead M. M. Haddadin M. J. J. Org. Chem. 2005;70:1060. doi: 10.1021/jo048153i. - DOI - PubMed
- Correa A. Tellitu I. Domınguez E. SanMartin R. Tetrahedron. 2006;62:11100. doi: 10.1016/j.tet.2006.09.031. - DOI
- Correa A. Tellitu I. Dominguez E. SanMartin R. J. Org. Chem. 2006;71:3501. doi: 10.1021/jo060070+. - DOI - PubMed
- Sawant D. Kumar R. Maulik P. R. Kundu B. Org. Lett. 2006;8:1525. doi: 10.1021/ol053033y. - DOI - PubMed
- Counceller C. M. Eichman C. C. Wray B. C. Stambuli J. P. Org. Lett. 2008;10:1021. doi: 10.1021/ol800053f. - DOI - PubMed
- Wang K. Fu X. Liu J. Liang Y. Dong D. Org. Lett. 2009;11:1015. doi: 10.1021/ol802952e. - DOI - PubMed
- Lehmann F. Koolmeister T. Odell L. R. Scobie M. Org. Lett. 2009;11:5078. doi: 10.1021/ol902085k. - DOI - PubMed
- Wray B. C. Stambuli J. P. Org. Lett. 2010;12:4576. doi: 10.1021/ol101899q. - DOI - PubMed
- Hu J. Cheng Y. Yang Y. Rao Y. Chem. Commun. 2011;47:10133. doi: 10.1039/C1CC13908H. - DOI - PubMed
- Yu D.-G. Suri M. Glorius F. J. Am. Chem. Soc. 2013;135:8802. doi: 10.1021/ja4033555. - DOI - PubMed
- Lin W.-C. Yang D.-Y. Org. Lett. 2013;15:4862. doi: 10.1021/ol402286d. - DOI - PubMed
- Park S. W. Choi H. Lee J.-h. Lee Y.-J. Ku J.-M. Lee S. Y. Nam T.-g. Arch. Pharm. Res. 2016;39:302. doi: 10.1007/s12272-016-0706-z. - DOI - PubMed
- Dai G. Yang L. Zhou W. Org. Chem. Front. 2017;4:229. doi: 10.1039/C6QO00573J. - DOI
-
For a review on intramolecular N–N bond formation, see:
- Guo Q. Lu Z. Synthesis. 2017;49:3835. doi: 10.1055/s-0036-1588512. - DOI
- Zhang Y. Duan D. Zhong Y. Guo X.-A. Guo J. Gou J. Gao Z. Yu B. Org. Lett. 2019;21:4960. doi: 10.1021/acs.orglett.9b01396. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

