Photodriven water oxidation initiated by a surface bound chromophore-donor-catalyst assembly
- PMID: 34880995
- PMCID: PMC8580115
- DOI: 10.1039/d1sc03896f
Photodriven water oxidation initiated by a surface bound chromophore-donor-catalyst assembly
Abstract
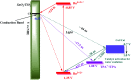
In photosynthesis, solar energy is used to produce solar fuels in the form of new chemical bonds. A critical step to mimic photosystem II (PS II), a key protein in nature's photosynthesis, for artificial photosynthesis is designing devices for efficient light-driven water oxidation. Here, we describe a single molecular assembly electrode that duplicates the key components of PSII. It consists of a polypyridyl light absorber, chemically linked to an intermediate electron donor, with a molecular-based water oxidation catalyst on a SnO2/TiO2 core/shell electrode. The synthetic device mimics PSII in achieving sustained, light-driven water oxidation catalysis. It highlights the value of the tyrosine-histidine pair in PSII in achieving efficient water oxidation catalysis in artificial photosynthetic devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Tachibana Y. Vayssieres L. Durrant J. R. Nat. Photonics. 2012;6:511–518. doi: 10.1038/nphoton.2012.175. - DOI
LinkOut - more resources
Full Text Sources

