Improvement of the antimicrobial potency, pharmacokinetic and pharmacodynamic properties of albicidin by incorporation of nitrogen atoms
- PMID: 34881013
- PMCID: PMC8580050
- DOI: 10.1039/d1sc04019g
Improvement of the antimicrobial potency, pharmacokinetic and pharmacodynamic properties of albicidin by incorporation of nitrogen atoms
Abstract
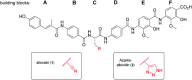
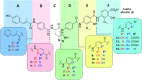
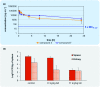
The worrisome development and spread of multidrug-resistant bacteria demands new antibacterial agents with strong bioactivities particularly against Gram-negative bacteria. Albicidins were recently structurally characterized as highly active antibacterial natural products from the bacterium Xanthomonas albilineans. Albicidin, which effectively targets the bacterial DNA-gyrase, is a lipophilic hexapeptide mostly consisting of para amino benzoic acid units and only one α-amino acid. In this study, we report on the design and synthesis of new albicidins, containing N-atoms on each of the 5 different phenyl rings. We systematically introduced N-atoms into the aromatic backbone to monitor intramolecular H-bonds and for one derivative correlated them with a significant enhancement of the antibacterial activity and activity spectrum, particularly also towards Gram-positive bacteria. In parallel we conducted DFT calculations to find the most stable conformation of each derivative. A drastic angle-change was observed for the lead compound and shows a preferred planarity through H-bonding with the introduced N-atom at the D-fragment of albicidin. Finally, we went to the next level and conducted the first in vivo experiments with an albicidin analogue. Our lead compound was evaluated in two different mouse experiments: In the first we show a promising PK profile and the absence of toxicity and in the second very good efficiency and reduction of the bacterial titre in an E. coli infection model with FQ-resistant clinically relevant strains. These results qualify albicidins as active antibacterial substances with the potential to be developed as a drug for treatment of infections caused by Gram-negative and Gram-positive bacteria.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
FOG is employee of Gombert Pharma Research Solutions (GPRS) and shareholder of Selmod LLC. The other authors declare no conflict of interest. Patents are filed.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

