Gut-Evolved Candida albicans Induces Metabolic Changes in Neutrophils
- PMID: 34881192
- PMCID: PMC8645939
- DOI: 10.3389/fcimb.2021.743735
Gut-Evolved Candida albicans Induces Metabolic Changes in Neutrophils
Abstract
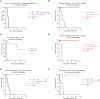
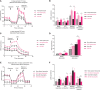
Serial passaging of the human fungal pathogen Candida albicans in the gastrointestinal tract of antibiotics-treated mice selects for virulence-attenuated strains. These gut-evolved strains protect the host from infection by a wide range of pathogens via trained immunity. Here, we further investigated the molecular and cellular mechanisms underlying this innate immune memory. Both Dectin-1 (the main receptor for β-glucan; a well-described immune training molecule in the fungal cell wall) and Nod2 (a receptor described to mediate BCG-induced trained immunity), were redundant for the protection induced by gut-evolved C. albicans against a virulent C. albicans strain, suggesting that gut-evolved C. albicans strains induce trained immunity via other pathways. Cytometry by time of flight (CyTOF) analysis of mouse splenocytes revealed that immunization with gut-evolved C. albicans resulted in an expansion of neutrophils and a reduction in natural killer (NK) cells, but no significant numeric changes in monocytes, macrophages or dendritic cell populations. Systemic depletion of phagocytes or neutrophils, but not of macrophages or NK cells, reduced protection mediated by gut-evolved C. albicans. Splenocytes and bone marrow cells of mice immunized with gut-evolved C. albicans demonstrated metabolic changes. In particular, splenic neutrophils displayed significantly elevated glycolytic and respiratory activity in comparison to those from mock-immunized mice. Although further investigation is required for fully deciphering the trained immunity mechanism induced by gut-evolved C. albicans strains, this data is consistent with the existence of several mechanisms of trained immunity, triggered by different training stimuli and involving different immune molecules and cell types.
Keywords: Candida albicans; immunometabolism; infectious diseases; live attenuated vaccines; neutrophils; trained immunity.
Copyright © 2021 Reales-Calderon, Tso, Tan, Hor, Böhme, Teng, Newell, Singhal and Pavelka.
Conflict of interest statement
EN is a co-founder, advisor and shareholder of ImmunoScape Pte. Ltd. EN is an advisor for Neogene Therapeutics and Nanostring Technologies. NP is employed by F. Hoffmann-La Roche AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

