Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species
- PMID: 34881193
- PMCID: PMC8647967
- DOI: 10.3389/fcimb.2021.757718
Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species
Abstract
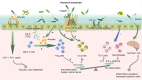
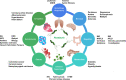
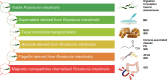
Roseburia intestinalis is an anaerobic, Gram-positive, slightly curved rod-shaped flagellated bacterium that produces butyrate in the colon. R. intestinalis has been shown to prevent intestinal inflammation and maintain energy homeostasis by producing metabolites. Evidence shows that this bacterium contributes to various diseases, such as inflammatory bowel disease, type 2 diabetes mellitus, antiphospholipid syndrome, and atherosclerosis. This review reveals the potential therapeutic role of R. intestinalis in human diseases. Patients with inflammatory bowel disease exhibit significant changes in R. intestinalis abundance, and they may benefit a lot from modulations targeting R. intestinalis. The data reviewed here demonstrate that R. intestinalis plays its role in regulating barrier homeostasis, immune cells, and cytokine release through its metabolite butyrate, flagellin and other. Recent advancements in the application of primary culture technology, culture omics, single-cell sequencing, and metabonomics technology have improved research on Roseburia and revealed the benefits of this bacterium in human health and disease treatment.
Keywords: Roseburia; Roseburia intestinalis; inflammatory bowel disease (IBD); microbiome; probiotic.
Copyright © 2021 Nie, Ma, Luo, Shen, Yang, Xiao, Tong, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bajaj J. S., Hylemon P. B., Ridlon J. M., Heuman D. M., Daita K., White M. B., et al. . (2012). Colonic Mucosal Microbiome Differs From Stool Microbiome in Cirrhosis and Hepatic Encephalopathy and is Linked to Cognition and Inflammation. Am. J. Physiol. Gastrointest Liver Physiol. 303 (6), G675–G685. doi: 10.1152/ajpgi.00152.2012 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

