Flow cytometric minimal residual disease assessment in B-cell precursor acute lymphoblastic leukaemia patients treated with CD19-targeted therapies - a EuroFlow study
- PMID: 34881427
- PMCID: PMC9299641
- DOI: 10.1111/bjh.17992
Flow cytometric minimal residual disease assessment in B-cell precursor acute lymphoblastic leukaemia patients treated with CD19-targeted therapies - a EuroFlow study
Abstract
The standardized EuroFlow protocol, including CD19 as primary B-cell marker, enables highly sensitive and reliable minimal residual disease (MRD) assessment in B-cell precursor acute lymphoblastic leukaemia (BCP-ALL) patients treated with chemotherapy. We developed and validated an alternative gating strategy allowing reliable MRD analysis in BCP-ALL patients treated with CD19-targeting therapies. Concordant data were obtained in 92% of targeted therapy patients who remained CD19-positive, whereas this was 81% in patients that became (partially) CD19-negative. Nevertheless, in both groups median MRD values showed excellent correlation with the original MRD data, indicating that, despite higher interlaboratory variation, the overall MRD analysis was correct.
Keywords: acute leukaemia; diagnostic haematology; flow cytometry; minimal residual disease.
© 2021 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
JJMvD, AO, JFM, TS and VHJvdV each report being one of the inventors on the EuroFlow‐owned patent PCT/NL2010/050332 (Methods, reagents and kits for flow cytometric immunophenotyping of normal, reactive and malignant leukocytes). The Infinicyt software is based on intellectual property of some EuroFlow laboratories (University of Salamanca in Spain and Federal University of Rio de Janeiro in Brazil) and the scientific input of other EuroFlow members. All above‐mentioned intellectual property and related patents are licensed to Cytognos (Salamanca, Spain) and BD Biosciences (San José, CA, USA), which companies pay royalties to the EuroFlow Consortium. These royalties are exclusively used for continuation of the EuroFlow collaboration and sustainability of the EuroFlow consortium. VHJvdV reports a Laboratory Services Agreement with BD Biosciences; all related fees are for the Erasmus MC. JJMvD and JAOMCV report an Educational Services Agreement from BD Biosciences and a Scientific Advisor Agreement with Cytognos; all related fees and honoraria are for the involved university departments at Leiden University Medical Centre and University of Salamanca. MB received personal fees from Incyte (advisory board) and Roche Pharma AG, financial support for reference diagnostics from Affimed, Amgen, BMS and Regeneron, grants and personal fees from Amgen (advisory board, speakers bureau, travel support), and personal fees from Janssen (speakers bureau), all outside the submitted work. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
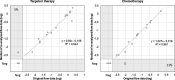
References
-
- van der Velden VHJ, Panzer‐Grümayer ER, Cazzaniga G, Flohr T, Sutton R, Schrauder A, et al. Optimization of PCR‐based minimal residual disease diagnostics for childhood acute lymphoblastic leukemia in a multi‐center setting. Leukemia. 2007;21(4):706–13. - PubMed
-
- Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–74. - PubMed
-
- Ribera J‐M, Morgades M, Ciudad J, Montesinos P, Esteve J, Genescà E, et al. Chemotherapy or allogeneic transplantation in high‐risk Philadelphia chromosome‐negative adult lymphoblastic leukemia. Blood. 2021;137(14):1879–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

