Synthesis of α-Branched Amines by Three- and Four-Component C-H Functionalization Employing a Readily Diversifiable Hydrazone Directing Group
- PMID: 34881902
- PMCID: PMC8785212
- DOI: 10.1021/acs.orglett.1c03807
Synthesis of α-Branched Amines by Three- and Four-Component C-H Functionalization Employing a Readily Diversifiable Hydrazone Directing Group
Abstract
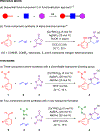
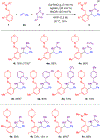
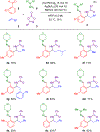
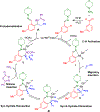
Efficient syntheses of α-branched amines by three- and four-component C-H functionalization employing a diversifiable hydrazone directing group have been developed. The hydrazone in the α-branched amine products has been readily converted to multiple desirable functionalities such as a nitrile, a carboxylic acid, alkenes, and heterocycles using diverse heterolytic chemistry and homolytic transition metal- or photoredox-catalyzed processes. This study represents the first example of a four-component C-H functionalization reaction.
Figures


References
-
- For examples of three-component C–H functionalization reactions employing Rh(III) catalysis, see: (a) Boerth JA; Ellman JA. Rh(III)-Catalyzed Diastereoselective C–H Bond Addition/Cyclization Cascade of Enone Tethered Aldehydes. Chem. Sci. 2016, 7, 1474–1479. - PMC - PubMed
- Zhang S-S; Xia J; Wu J-Q; Liu X-G; Zhou C-J; Lin E; Li Q; Huang S-L; Wang H. Three-Component Catalytic Carboxygenation of Activated Alkenes Enabled by Bimetallic Rh(III)/Cu(II) Catalysis. Org. Lett. 2017, 19, 5868–5871. - PMC - PubMed
- Tang M; Li Y; Han S; Liu L; Ackermann L; Li J. Rhodium(III)-Catalyzed C–H Alkylation/Nucleophilic Addition Domino Reaction. Eur. J. Org. Chem. 2019, 660–664. - PMC - PubMed
- Li R; Ju C-W; Zhao D. Rhodium(III) vs. Cobalt(III): A Mechanistically Distinct Three-Component C–H Bond Addition Cascade Using a Cp*RhIII Catalyst. Chem. Commun. 2019, 55, 695–698. - PMC - PubMed
- Maity S; Potter TJ; Ellman JA α-Branched Amines by Catalytic 1,1-Addition of C–H Bonds and Aminating Agents to Terminal Alkenes. Nat. Catal. 2019, 2, 756–762. - PMC - PubMed
- Pinkert T; Wegner T; Mondal S; Glorius F. Intermolecular 1,4-Carboamination of Conjugated Dienes Enabled by Cp*RhIII-Catalyzed C−H Activation. Angew. Chem., Int. Ed. 2019, 58, 15041–15045. - PMC - PubMed
- Brandes DS; Sirvent A; Mercado BQ; Ellman JA Three-Component 1,2-Carboamidation of Bridged Bicyclic Alkenes via RhIII-Catalyzed Addition of C–H Bonds and Amidating Reagents. Org. Lett. 2021, 23, 2836–2840. - PMC - PubMed
- Mi R; Zhang X; Wang J; Chen H; Lan Y; Wang F; Li X. Rhodium-Catalyzed Regio-, Diastereo-, and Enantioselective Three-Component Carboamination of Dienes via C–H Activation. ACS Catal. 2021, 11, 6692–6697. - PMC - PubMed
- Pinkert T; Das M; Schrader ML; Glorius F. Use of Strain-Release for the Diastereoselective Construction of Quaternary Carbon Centers. J. Am. Chem. Soc. 2021, 143, 7648–7654. - PMC - PubMed
-
- For examples of three-component C–H functionalization reactions employing Co(III) catalysis, see: (a) Boerth JA; Hummel JR; Ellman JA. Highly Stereoselective Cobalt(III)-Catalyzed Three-Component C−H Bond Addition Cascade. Angew. Chem., Int. Ed. 2016, 55, 12650–12654. - PMC - PubMed
- Boerth JA; Ellman JA A Convergent Synthesis of Functionalized Alkenyl Halides through Cobalt(III)-Catalyzed Three-Component C−H Bond Addition. Angew. Chem., Int. Ed. 2017, 56, 9976–9980. - PMC - PubMed
- Boerth JA; Maity S; Williams SK; Mercado BQ; Ellman JA Selective and Synergistic Cobalt(III)-Catalysed Three-Component C–H Bond Addition to Dienes and Aldehydes. Nat. Catal. 2018, 1, 673–679. - PMC - PubMed
- Dongbang S; Shen Z; Ellman JA Synthesis of Homoallylic Alcohols with Acyclic Quaternary Centers through CoIII-Catalyzed Three-Component C−H Bond Addition to Internally Substituted Dienes and Carbonyls. Angew. Chem., Int. Ed. 2019, 58, 12590–12594. - PMC - PubMed
- Yang J; Ji D-W; Hu Y-C; Min X-T; Zhou X; Chen Q-A Cobalt-Catalyzed Hydroxymethylarylation of Terpenes with Formaldehyde and Arenes. Chem. Sci. 2019, 10, 9560–9564. - PMC - PubMed
- Shen Z; Li C; Mercado BQ; Ellman JA Cobalt(III)-Catalyzed Diastereoselective Three-Component C–H Bond Addition to Butadiene and Activated Ketones. Synthesis 2020, 52, 1239–1246. - PMC - PubMed
- Dongbang S; Ellman JA Synthesis of Nitrile Bearing Acyclic Quaternary Centers through Co(III)-Catalyzed Sequential C−H Bond Addition to Dienes and N-Cyanosuccinimide. Angew. Chem., Int. Ed. 2021, 60, 2135–2139. - PMC - PubMed
- Herraiz AG; Cramer N. Cobalt(III)-Catalyzed Diastereo- and Enantioselective Three-Component C–H Functionalization. ACS Catal. 2021, 11, 11938–11944. - PMC - PubMed
-
- For an example of three-component C–H functionalization reactions employing Ru(II) catalysis, see: Wang X-G; Li Y; Liu H-C; Zhang B-S; Gou X-Y; Wang Q; Ma J-W; Liang Y-M. Three-Component Ruthenium-Catalyzed Direct Meta-Selective C–H Activation of Arenes: A New Approach to the Alkylarylation of Al-kenes. J. Am. Chem. Soc. 2019, 141, 13914–13922. - PubMed
-
- Nugent TC Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH GmbH & Co., Weinheim, 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

