Chemotherapy for second-stage human African trypanosomiasis: drugs in use
- PMID: 34882307
- PMCID: PMC8656462
- DOI: 10.1002/14651858.CD015374
Chemotherapy for second-stage human African trypanosomiasis: drugs in use
Abstract
Background: Human African trypanosomiasis, or sleeping sickness, is a severe disease affecting people in the poorest parts of Africa. It is usually fatal without treatment. Conventional treatments require days of intravenous infusion, but a recently developed drug, fexinidazole, can be given orally. Another oral drug candidate, acoziborole, is undergoing clinical development and will be considered in subsequent editions. OBJECTIVES: To evaluate the effectiveness and safety of currently used drugs for treating second-stage Trypanosoma brucei gambiense trypanosomiasis (gambiense human African trypanosomiasis, g-HAT).
Search methods: On 14 May 2021, we searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, Latin American and Caribbean Health Science Information database, BIOSIS, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform. We also searched reference lists of included studies, contacted researchers working in the field, and contacted relevant organizations.
Selection criteria: Eligible studies were randomized controlled trials that included adults and children with second-stage g-HAT, treated with anti-trypanosomal drugs currently in use.
Data collection and analysis: Two review authors extracted data and assessed risk of bias; a third review author acted as an arbitrator if needed. The included trial only reported dichotomous outcomes, which we presented as risk ratio (RR) or risk difference (RD) with 95% confidence intervals (CI). MAIN RESULTS: We included one trial comparing fexinidazole to nifurtimox combined with eflornithine (NECT). This trial was conducted between October 2012 and November 2016 in the Democratic Republic of the Congo and the Central African Republic, and included 394 participants. The study reported on efficacy and safety, with up to 24 months' follow-up. We judged the study to be at low risk of bias in all domains except blinding; as the route of administration and dosing regimens differed between treatment groups, participants and personnel were not blinded, resulting in a high risk of performance bias. Mortality with fexinidazole may be higher at 24 months compared to NECT. There were 9/264 deaths in the fexinidazole group and 2/130 deaths in the NECT group (RR 2.22, 95% CI 0.49 to 10.11; 394 participants; low-certainty evidence). None of the deaths were related to treatment. Fexinidazole likely results in an increase in the number of people relapsing during follow-up, with 14 participants in the fexinidazole group (14/264) and none in the NECT group (0/130) relapsing at 24 months (RD 0.05, 95% CI 0.02 to 0.08; 394 participants; moderate-certainty evidence). We are uncertain whether there is any difference between the drugs regarding the incidence of serious adverse events at 24 months. (31/264 with fexinidazole and 13/130 with NECT group at 24 months). Adverse events were common with both drugs (247/264 with fexinidazole versus 121/130 with NECT), with no difference between groups (RR 1.01, 95% CI 0.95 to 1.06; 394 participants; moderate-certainty evidence). AUTHORS' CONCLUSIONS: Oral treatment with fexinidazole is much easier to administer than conventional treatment, but deaths and relapse appear to be more common. However, the advantages or an oral option are considerable, in terms of convenience, avoiding hospitalisation and multiple intravenous infusions, thus increasing adherence.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
VL has no known conflicts of interest. VL works as an independent consultant conducting literature searches for various research groups. None of them has any potential relevance to the submitted work.
KP received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
JS has participated as an expert in the WHO guideline development group for the use of fexinidazole; collaborates in the WHO network for Human African Trypanosomiasis Elimination Group; is designated chairman of the subgroup 'Integration of new tools into national and global policies' of the aforementioned WHO Group; is designated member of the WHO Human African Trypanosomiasis Elimination Technical Advisory Group (HAT‐e‐TAG); and participated in 2018 as an expert in the Scientific Advisory Group meeting on Fexinidazole Winthrop, promoted by the Committee for Medicinal Products for Human Use of the European Medicines Agency.
HB received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
GV received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Figures
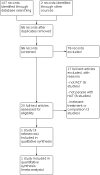









Similar articles
-
Chemotherapy for second-stage human African trypanosomiasis.Cochrane Database Syst Rev. 2013 Jun 28;2013(6):CD006201. doi: 10.1002/14651858.CD006201.pub3. Cochrane Database Syst Rev. 2013. PMID: 23807762 Free PMC article.
-
Chemotherapy for second-stage Human African trypanosomiasis.Cochrane Database Syst Rev. 2010 Aug 4;(8):CD006201. doi: 10.1002/14651858.CD006201.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 28;(6):CD006201. doi: 10.1002/14651858.CD006201.pub3. PMID: 20687080 Updated.
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Heliox for croup in children.Cochrane Database Syst Rev. 2021 Aug 16;8(8):CD006822. doi: 10.1002/14651858.CD006822.pub6. Cochrane Database Syst Rev. 2021. PMID: 34397099 Free PMC article.
Cited by
-
Polyamine Metabolism for Drug Intervention in Trypanosomatids.Pathogens. 2024 Jan 16;13(1):79. doi: 10.3390/pathogens13010079. Pathogens. 2024. PMID: 38251386 Free PMC article. Review.
-
Bis-6-amidino-benzothiazole Derivative that Cures Experimental Stage 1 African Trypanosomiasis with a Single Dose.J Med Chem. 2023 Sep 28;66(18):13043-13057. doi: 10.1021/acs.jmedchem.3c01051. Epub 2023 Sep 18. J Med Chem. 2023. PMID: 37722077 Free PMC article.
-
Antimicrobial Peptides (AMPs): Potential Therapeutic Strategy against Trypanosomiases?Biomolecules. 2023 Mar 26;13(4):599. doi: 10.3390/biom13040599. Biomolecules. 2023. PMID: 37189347 Free PMC article. Review.
-
Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates.Biomolecules. 2023 Apr 1;13(4):637. doi: 10.3390/biom13040637. Biomolecules. 2023. PMID: 37189384 Free PMC article. Review.
-
Discovery of an orally active nitrothiophene-based antitrypanosomal agent.Eur J Med Chem. 2024 Jan 5;263:115954. doi: 10.1016/j.ejmech.2023.115954. Epub 2023 Nov 15. Eur J Med Chem. 2024. PMID: 37984297 Free PMC article.
References
References to studies included in this review
Mesu 2018 {published and unpublished data}
-
- DNDi. Follow-up abbreviated clinical study report DNDiFEX004 - fexinidazole. Efficacy and safety of fexinidazole compared to nifurtimox-eflornithine combination therapy (NECT) in patients with late-stage human African trypanosomiasis (HAT) due to T. b. gambiense: a pivotal, non-inferiority, randomised, open-label, multicentre study. Data on file Unpublished clinical study report received from Drs Olaf Valverde Mordt and Antoine Tarral (DNDi) July 2019.
-
- European Medicines Agency Committee for Medicinal Products for Human Use. Assessment report fexinidazole winthrop; 2018. www.ema.europa.eu/en/documents/medicine-outside-eu/fexinidazole-winthrop... (accessed 16 November 2018).
-
- Mesu VK, Kalonji WM, Bardonneau C, Mordt OV, Blesson S, Simon F, et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet 2018;391(10116):144-54. - PubMed
References to studies excluded from this review
Alirol 2013 {published data only}
-
- Alirol E, Schrumpf D, Amici Heradi J, Riedel A, Patoul C, Quere M, et al. Nifurtimox-eflornithine combination therapy for second-stage gambiense human African trypanosomiasis: Medecins Sans Frontieres experience in the Democratic Republic of the Congo. Clinical Infectious Diseases 2013;56(2):195-203. - PubMed
Chappuis 2018 {published data only}
-
- Chappuis F. Oral fexinidazole for human African trypanosomiasis. Lancet 2018;391(10116):100-2. - PubMed
Jansson‐Löfmark 2015 {published data only}
-
- Jansson-Löfmark R, Na-Bangchang K, Björkman S, Doua F, Ashton M. Enantiospecific reassessment of the pharmacokinetics and pharmacodynamics of oral eflornithine against late-stage Trypanosoma brucei gambiense sleeping sickness. Antimicrobial Agents and Chemotherapy 2015;59(2):1299-307. - PMC - PubMed
Kansiime 2018 {published data only}
-
- Kansiime F, Adibaku S, Wamboga C, Idi F, Kato CD, Yamuah L, et al. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasites & Vectors 2018;11(1):105. - PMC - PubMed
Kazumba 2018 {published data only}
Mord 2013 {published data only}
-
- Mord OV, Bernhard S, Ghabri S, Kande V, Mutombo W, Ilunga M, et al. NECT feld phase IIIb trial: final effectiveness in adults and children results. Tropical Medicine & International Health 2013;18(Suppl 1):59-60.
NCT00982904 {published data only}
-
- NCT00982904. Human African trypanosomiasis: first in man clinical trial of a new medicinal product, the fexinidazole [Randomized, double-blind, placebo-controlled study of the tolerability, and pharmacokinetics of fexinidazole after single and repeated oral ascending doses, completed by a comparative bioavailability study of an oral suspension versus a tablet and an exploratory assessment of food effect, in healthy male volunteers]. clinicaltrials.gov/ct2/show/NCT00982904 (first posted 23 September 2009).
NCT01340157 {published data only}
-
- NCT01340157. Fexinidazole (1200mg) bioavailability under different food intake conditions [Randomized, open study to assess the impact of two different types of food on the relative bioavailability of fexinidazole tablets after single oral dose in healthy male volunteers]. clinicaltrials.gov/ct2/show/NCT01340157 (first posted 22 April 2011).
NCT01483170 {published data only}
-
- NCT01483170. Multiple dose study to evaluate security, tolerance and pharmacokinetic of fexinidazole (drug candidate for human African trypanosomiasis) administered with a loading dose and with food [Double-blind, placebo controlled, randomized multiple ascending dose study in fed conditions for ten days dosing regimen with a loading dose to evaluate the safety, the tolerability and the pharmacokinetics of oral fexinidazole in 36 healthy male sub-Saharan volunteers]. clinicaltrials.gov/ct2/show/NCT01483170 (first posted 1 December 2011).
NCT02571062 {published data only}
-
- NCT02571062. Bioequivalence study - reference clinical fexinidazole tablet versus proposed market formulation [A bioequivalence study of the reference clinical fexinidazole tablet vs proposed market formulation in healthy male volunteers of African sub-Saharan origin: an open-label, randomized, two-treatment, single dose, replicate design, fed condition]. clinicaltrials.gov/ct2/show/NCT02571062 (first posted 8 October 2015).
Pelfrene 2019 {published data only}
Pollastri 2018 {published data only}
Schmid 2012 {published data only}
Tarral 2011 {published data only}
-
- Tarral A, Valverde O, Blesson S, Strub-Wourgaft N, Hosvepian L, Evene E. Single-dose safety, pharmacokinetics (PK) and pharmacodynamics (PD) of fexinidazole in healthy subjects. 7th European Congress on Tropical Medicine & International Health. 2011. www.dndi.org/media-centre/events/events-2011/216-media-centre/events/980... note: authors please check link as it's no longer valid. Do you mean https://dndi.org/wp-content/uploads/2011/10/Fexi%20Antoine%20FINAL.pdf?) (accesssed dd Month yyyy).
Tarral 2014 {published data only}
Valverde 2015 {published data only}
-
- Valverde Mordt O, Bernhard S, Ghabri S, Kande V, Mutombo W, Ilunga M, et al. Nifurtimox eflornithine combination therapy phase IIIb field trial (NECT Field): final effectiveness and safety results. Tropical Medicine & International Health 2015;20(Suppl 1):325.
Watson 2019 {published data only}
References to ongoing studies
NCT03087955 {published and unpublished data}
-
- NCT03087955. Prospective study on efficacy and safety of acoziborole (SCYX-7158) in patients infected by human African trypanosomiasis due to T. b. gambiense (OXA002) [Efficacy and safety study of acoziborole (SCYX-7158) in patients with human African trypanosomiasis (HAT) due to Trypanosoma brucei gambiense: a multicentre, open-label, prospective study]. clinicaltrials.gov/ct2/show/NCT03087955 (first posted 23 March 2017).
Additional references
Altman 1998
Barrett 2018
Brun 2010
-
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet 2010;375(9709):148-59. - PubMed
Büscher 2017
-
- Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet 2017;390(10110):2397-409. - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 10 January 2017. Available at covidence.org.
Eperon 2014
Franco 2012
Franco 2014
-
- Franco JR, Simarro PP, Diarra A, Ruiz-Postigo JA, Jannin JG. The journey towards elimination of gambiense human African trypanosomiasis: not far, nor easy. Parasitology 2014;141(6):748-60. - PubMed
Franco 2017
Franco 2018
Franco 2020
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed before 23 November 2021. Available at gradepro.org.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019.
Jones 2015
Kennedy 2019
Lejon 2005
-
- Lejon V, Büscher P. Review Article: cerebrospinal fluid in human African trypanosomiasis: a key to diagnosis, therapeutic decision and post-treatment follow-up. Tropical Medicine & International Health 2005;10(5):395-403. - PubMed
Lindner 2020
-
- Lindner AK, Lejon V, Chappuis F, Seixas J, Kazumba L, Barrett MP, et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. Lancet Infectious Diseases 2020;20(2):e38-46. - PubMed
Mesu 2021
-
- Mesu VK, Kalonji WM, Bardonneau C, Mordt OV, Digas NT, Blesson S, et al. Oral fexinidazole for stage 1 or early stage 2 African Trypanosoma brucei gambiense trypanosomiasis: a prospective, multicentre, open-label, cohort study. Lancet Global Health 2021;9(7):e999-1008. [DOI: 10.1016/S2214-109X(21)00208-4] - DOI - PMC - PubMed
NCT01533961
-
- NCT01533961. Human African trypanosomiasis: first in man clinical trial of a new medicinal product, the SCYX-7158 [Randomized, double-blind, placebo-controlled sequential study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of SCYX-7158 after single oral ascending doses in healthy male volunteers]. clinicaltrials.gov/ct2/show/NCT01533961 (first posted 16 February 2012).
Pépin 2001
-
- Pépin J, Méda HA. The epidemiology and control of human African trypanosomiasis. Advances in Parasitology 2001;49:71-132. - PubMed
Priotto 2009
-
- Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 2009;374(9683):56-64. - PubMed
RevMan 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Seed 2001
-
- Seed JR, Boykin DW. Chemotherapy of African trypanosomiasis. In: Black SJ, Seed JR, editors(s). The African trypanosomes. Series: World Class Parasites, Vol. 1. Boston: Kluwer Academic Publishers, 2001:65-78.
Stich 2002
Torreele 2010
WHO 2004
-
- World Health Organization. Recommendations of the informal consultation on issue for clinical product development for human African trypanosomiasis; Geneva, Switzerland. 9-10 September 2004. Available at www.who.int/neglected_diseases/mediacentre/Final%20Clinical_products_for.... [WHO/CDS/NTD/IDM/2007.1]
WHO 2010
-
- World Health Organization. WHO model list of essential medicines, 16th list (updated); March 2010. Available at www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_....
WHO 2012
-
- World Health Organization. Accelerating work to overcome neglected tropical diseases: a roadmap for implementation; 2012. Available at www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf.
WHO 2013
-
- World Health Organization. Report of a WHO meeting on elimination of African trypanosomiasis (Trypanosoma brucei gambiense); 2013. who.int/publications/i/item/WHO-HTM-NTD-IDM-2013.4.
WHO 2019
-
- World Health Organization. WHO interim guidelines for the treatment of gambiense human African trypanosomiasis; 2019. apps.who.int/iris/bitstream/handle/10665/326178/9789241550567-eng.pdf?ua=1. - PubMed
References to other published versions of this review
Lutje 2006
Lutje 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

