Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans - Five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021
- PMID: 34882654
- PMCID: PMC8659185
- DOI: 10.15585/mmwr.mm7049a2
Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans - Five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021
Abstract
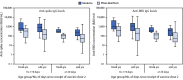
The mRNA COVID-19 vaccines (Moderna and Pfizer-BioNTech) provide strong protection against severe COVID-19, including hospitalization, for at least several months after receipt of the second dose (1,2). However, studies examining immune responses and differences in protection against COVID-19-associated hospitalization in real-world settings, including by vaccine product, are limited. To understand how vaccine effectiveness (VE) might change with time, CDC and collaborators assessed the comparative effectiveness of Moderna and Pfizer-BioNTech vaccines in preventing COVID-19-associated hospitalization at two periods (14-119 days and ≥120 days) after receipt of the second vaccine dose among 1,896 U.S. veterans at five Veterans Affairs medical centers (VAMCs) during February 1-September 30, 2021. Among 234 U.S. veterans fully vaccinated with an mRNA COVID-19 vaccine and without evidence of current or prior SARS-CoV-2 infection, serum antibody levels (anti-spike immunoglobulin G [IgG] and anti-receptor binding domain [RBD] IgG) to SARS-CoV-2 were also compared. Adjusted VE 14-119 days following second Moderna vaccine dose was 89.6% (95% CI = 80.1%-94.5%) and after the second Pfizer-BioNTech dose was 86.0% (95% CI = 77.6%-91.3%); at ≥120 days VE was 86.1% (95% CI = 77.7%-91.3%) for Moderna and 75.1% (95% CI = 64.6%-82.4%) for Pfizer-BioNTech. Antibody levels were significantly higher among Moderna recipients than Pfizer-BioNTech recipients across all age groups and periods since vaccination; however, antibody levels among recipients of both products declined between 14-119 days and ≥120 days. These findings from a cohort of older, hospitalized veterans with high prevalences of underlying conditions suggest the importance of booster doses to help maintain long-term protection against severe COVID-19.†.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Vincent C. Marconi reports institutional support to Emory University from the AIDS Clinical Trials Group and the National Institutes of Health (NIH); grants from Lilly, Gilead, ViiV, NIH, and the Veterans Health Administration; payment or honoraria from Medscape, WebMD, ViiV, Integritas, and Lilly; travel support from NIH; and participation on an NIH Data Safety Monitoring Board. Miwako Kobayashi reports support for attending a meeting from the American Veterinary Medical Association. No other potential conflicts of interest were disclosed.
Figures
References
-
- Bajema KL, Dahl RM, Prill MM, et al.; SUPERNOVA COVID-19; Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five Veterans Affairs Medical Centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1294–9. 10.15585/mmwr.mm7037e3 - DOI - PMC - PubMed
-
- Tenforde MW, Self WH, Naioti EA, et al.; IVY Network Investigators; IVY Network. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19–associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156–62. 10.15585/mmwr.mm7034e2 - DOI - PMC - PubMed
-
- Meites E, Bajema KL, Kambhampati A, et al. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health 2021;9:739076. 10.3389/fpubh.2021.739076 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

