Growth hormone treatment does not to lead to insulin resistance nor excessive rise in IGF-1 levels, while improving height in patients small for gestational age A long-term observational study
- PMID: 34882803
- PMCID: PMC9299847
- DOI: 10.1111/cen.14626
Growth hormone treatment does not to lead to insulin resistance nor excessive rise in IGF-1 levels, while improving height in patients small for gestational age A long-term observational study
Abstract
Objective: In children born small for gestational age (SGA), the relationship between growth hormone (GH) treatment and insulin resistance (IR) has only been investigated for a short period, necessitating a longer observation period. This study aimed to evaluate the long-term (10 years) effect of GH to SGA-children on IR and safety during treatment.
Design: This was a multicenter observational study.
Patients: SGA-children who received GH treatment in Spain (stratified by Tanner-stage and age at GH onset [two groups: ≤6 years old or >6 years old]).
Measurements: The analysed variables (yearly measures) included auxologic, metabolic (insulin-like growth factor-1 (IGF-1), height velocity [HV], weight and homeostatic model assessment-IR [HOMA-IR]) and safety data. Data were collected prospectively (since the study approval: 2007) and retrospectively (since the initiation of GH treatment: 2005-2007).
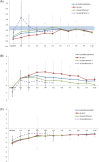
Results: A total of 389 SGA children (369 Tanner-I) were recruited from 27 centres. The mean age (standard deviation) of the children at GH treatment onset was 7.2 (2.8) years old. IGF-1 (standard deviation score [SDS]) and HOMA-IR values tended to increase until the sixth year of GH-treatment, with significant differences being observed only during the first year, while these remained stable in the later years (within normal ranges). Height (SDS) increased significantly (basal: -3.0; tenth year: -1.13), and the maximum HV (SDS) occurred during the first year (2.75 ± 2.39).
Conclusions: HOMA-IR values increased significantly in SGA-children during the first year of GH-treatment, remained stable and were within normal ranges in all cases. Our 10-year data suggests that long-term GH treatment does not promote IR and is well-tolerated, safe and effective.
Keywords: efficacy; growth hormone; homeostatic model assessment; insulin resistance; long-term follow-up; safety; small for gestational age.
© 2021 Merck Spain S.L.U. Clinical Endocrinology pusblished by John Wiley & Sons Ltd on behalf of Society for Endocrinology (SFE) and Clinical Endocrinology Trust (CET).
Conflict of interest statement
The authors belong to the Merck‐funded ‘SGA Research Collaborative Group6’. Bosh J. received honoraria as a lecturer from Merck, Lilly, Ipsen, Pfizer and Sandoz. Alfonso M. Lechuga‐Sancho is a consultant member of several promoted studies and an advisor to several pharmaceutical companies. Juan P. López‐Siguero has received honoraria as a lecturer and advisor from Merck, Sandoz and Lilly. Maria J. Martínez‐Aedo and Jose Antonio Bermúdez de la Vega do not declare any conflict of interests. Triana Villalobos is an employee from Merck, S.L.U.
Figures
References
-
- Lee PA, Chernausek SD, Hokken‐Koelega AC, Czernichow P, International SGA Advisory Board . International Small for Gestational Age Advisory Board Consensus Development ConferenceStatement: management of short children born small for gestational age, April 24‐October 1, 2001. Pediatrics. 2003;111(6 Pt 1):1253‐1261. 10.1542/peds.111.6.1253 - DOI - PubMed
-
- Arends NJ, Boonstra VH, Duivenvoorden HJ, Hofman PL, Cutfield WS, Hokken‐Koelega AC. Reduced insulin sensitivity and the presence of cardiovascular risk factors in short prepubertal children born small for gestational age (SGA). Clin Endocrinol. 2005;62(1):44‐50. 10.1111/j.1365-2265.2004.02171.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

