Piers' Borane-Induced Tetramerization of Arylacetylenes
- PMID: 34882876
- PMCID: PMC9303334
- DOI: 10.1002/chem.202104254
Piers' Borane-Induced Tetramerization of Arylacetylenes
Abstract
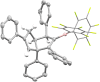
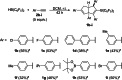
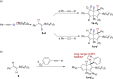
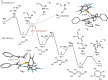
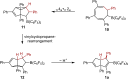
We herein report that the reaction of Piers' borane, i. e. HB(C6 F5 )2 , with an excess of arylacetylenes at room temperature leads to tetramerization of the acetylene and the diastereoselective formation of boryl-substituted tetra-aryl-tetrahydropentalenes. The reaction mechanism was investigated by isotope labeling experiments and DFT computations. These investigations indicate that a series of 1,2-carboboration reactions form an octatetraene that undergoes an electrocyclization. Two skeletal rearrangements then presumably lead to the formation of the tetrahydropentalene core. Overall, this intricate and unprecedented transformation comprises five carbon-carbon bond formations in a single reaction.
Keywords: 1,2-carboboration; Lewis acids; alkynes; boranes; density functional theory.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
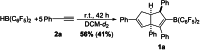

References
-
- None
-
- Miyaura N., Yamada K., Suzuki A., Tetrahedron Lett. 1979, 20, 3437–3440;
-
- Miyaura N., Suzuki A., Chem. Commun. 1979, 19, 866–867;
-
- Miyaura N., Suzuki A., Chem. Rev. 1995, 95, 2457–2483.
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

