Preoperative environment enrichment preserved neuroligin 1 expression possibly via epigenetic regulation to reduce postoperative cognitive dysfunction in mice
- PMID: 34882968
- PMCID: PMC8928916
- DOI: 10.1111/cns.13777
Preoperative environment enrichment preserved neuroligin 1 expression possibly via epigenetic regulation to reduce postoperative cognitive dysfunction in mice
Abstract
Aims: Postoperative cognitive dysfunction (POCD) is a common and significant syndrome. Our previous studies have shown that surgery reduces dendritic arborization and spine density and that environment enrichment (EE) reduces POCD. Neuroligin 1 is a postsynaptic protein involved in the formation of postsynaptic protein complex. This study was designed to determine the role of neuroligin 1 in the protection of EE against POCD and the mechanisms for EE to affect neuroligin 1 expression.
Methods: Eight-week-old C57BL/6J male mice with or without EE for 3, 7, or 14 days had right carotid artery exposure under isoflurane anesthesia. An anti-neuroligin 1 antibody at 1.5 µg/mouse was injected intracerebroventricularly at one and two weeks before the surgery. Mice were subjected to the Barnes maze and fear conditioning tests from one week after the surgery. Cerebral cortex and hippocampus were harvested after surgery.
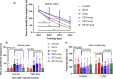
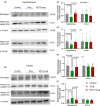
Results: Mice with surgery had poorer performance in the Barnes maze and fear conditioning tests than control mice. EE for 2 weeks, but not EE for 3 or 7 days, improved the performance of surgery mice in these tests. Surgery reduced neuroligin 1 in the hippocampus. Preoperative EE for 2 weeks attenuated this reduction. The anti-neuroligin 1 antibody worsened the performance of mice with surgery plus EE in the Barnes maze and fear conditioning tests. Surgery increased histone deacetylase activity and decreased the acetylated histone in the hippocampus. EE attenuated these surgery effects.
Conclusion: Our results suggest that preoperative EE for 2 weeks reduces POCD. This effect may be mediated by preserving neuroligin 1 expression via attenuating surgery-induced epigenetic dysregulation in the brain.
Keywords: environment enrichment; histone deacetylase; neuroligin 1; postoperative cognitive dysfunction.
© 2021 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Can Environmental Enrichment Modulate Epigenetic Processes in the Central Nervous System Under Adverse Environmental Conditions? A Systematic Review.Cell Mol Neurobiol. 2024 Oct 21;44(1):69. doi: 10.1007/s10571-024-01506-0. Cell Mol Neurobiol. 2024. PMID: 39432132 Free PMC article.
-
Role of Sox2 in Learning, Memory, and Postoperative Cognitive Dysfunction in Mice.Cells. 2021 Mar 24;10(4):727. doi: 10.3390/cells10040727. Cells. 2021. PMID: 33805206 Free PMC article.
-
Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice.J Neurochem. 2021 Jul;158(2):328-341. doi: 10.1111/jnc.15368. Epub 2021 May 5. J Neurochem. 2021. PMID: 33871050 Free PMC article.
-
Enriched Environment Attenuates Surgery-Induced Impairment of Learning, Memory, and Neurogenesis Possibly by Preserving BDNF Expression.Mol Neurobiol. 2016 Jan;53(1):344-354. doi: 10.1007/s12035-014-9013-1. Epub 2014 Nov 29. Mol Neurobiol. 2016. PMID: 25432890
-
Amantadine Alleviates Postoperative Cognitive Dysfunction Possibly by Preserving Neurotrophic Factor Expression and Dendritic Arborization in the Hippocampus of Old Rodents.Front Aging Neurosci. 2020 Nov 26;12:605330. doi: 10.3389/fnagi.2020.605330. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33324197 Free PMC article.
Cited by
-
Do We Have Measures to Reduce Post-operative Cognitive Dysfunction?Front Neurosci. 2022 Jun 2;16:850012. doi: 10.3389/fnins.2022.850012. eCollection 2022. Front Neurosci. 2022. PMID: 35720718 Free PMC article. No abstract available.
-
Co-housing with Alzheimer's disease mice induces changes in gut microbiota and impairment of learning and memory in control mice.CNS Neurosci Ther. 2024 Apr;30(4):e14491. doi: 10.1111/cns.14491. Epub 2023 Oct 3. CNS Neurosci Ther. 2024. PMID: 37789692 Free PMC article. No abstract available.
-
Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation.Cells. 2022 Sep 21;11(19):2954. doi: 10.3390/cells11192954. Cells. 2022. PMID: 36230916 Free PMC article. Review.
-
DNMT3a Deficiency Contributes to Anesthesia/Surgery-Induced Synaptic Dysfunction and Cognitive Impairment in Aged Mice.Aging Cell. 2025 Apr;24(4):e14458. doi: 10.1111/acel.14458. Epub 2024 Dec 25. Aging Cell. 2025. PMID: 39722450 Free PMC article.
-
Can Environmental Enrichment Modulate Epigenetic Processes in the Central Nervous System Under Adverse Environmental Conditions? A Systematic Review.Cell Mol Neurobiol. 2024 Oct 21;44(1):69. doi: 10.1007/s10571-024-01506-0. Cell Mol Neurobiol. 2024. PMID: 39432132 Free PMC article.
References
-
- Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18‐30. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548‐555. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Li Y, Chen D, Wang H, et al. Intravenous versus Volatile Anesthetic Effects on Postoperative Cognition in Elderly Patients Undergoing Laparoscopic Abdominal Surgery. Anesthesiology. 2021;134(3):381‐394. https://www.ncbi.nlm.nih.gov/pubmed/33439974 - PubMed
-
- Zheng B, Lai R, Li J, Zuo Z. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. Brain Behav Immun. 2017;61:365‐374. https://www.ncbi.nlm.nih.gov/pubmed/28089560 - PMC - PubMed
-
- Tian XS, Tong YW, Li ZQ, et al. Surgical stress induces brain‐derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci Ther. 2015;21(5):398‐409. https://www.ncbi.nlm.nih.gov/pubmed/25611431 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

