Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial
- PMID: 34883053
- PMCID: PMC8648333
- DOI: 10.1016/S0140-6736(21)02718-5
Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial
Erratum in
-
Department of Error.Lancet. 2022 Feb 26;399(10327):802. doi: 10.1016/S0140-6736(22)00321-X. Lancet. 2022. PMID: 35219392 Free PMC article. No abstract available.
Abstract
Background: Given the importance of flexible use of different COVID-19 vaccines within the same schedule to facilitate rapid deployment, we studied mixed priming schedules incorporating an adenoviral-vectored vaccine (ChAdOx1 nCoV-19 [ChAd], AstraZeneca), two mRNA vaccines (BNT162b2 [BNT], Pfizer-BioNTech, and mRNA-1273 [m1273], Moderna) and a nanoparticle vaccine containing SARS-CoV-2 spike glycoprotein and Matrix-M adjuvant (NVX-CoV2373 [NVX], Novavax).
Methods: Com-COV2 is a single-blind, randomised, non-inferiority trial in which adults aged 50 years and older, previously immunised with a single dose of ChAd or BNT in the community, were randomly assigned (in random blocks of three and six) within these cohorts in a 1:1:1 ratio to receive a second dose intramuscularly (8-12 weeks after the first dose) with the homologous vaccine, m1273, or NVX. The primary endpoint was the geometric mean ratio (GMR) of serum SARS-CoV-2 anti-spike IgG concentrations measured by ELISA in heterologous versus homologous schedules at 28 days after the second dose, with a non-inferiority criterion of the GMR above 0·63 for the one-sided 98·75% CI. The primary analysis was on the per-protocol population, who were seronegative at baseline. Safety analyses were done for all participants who received a dose of study vaccine. The trial is registered with ISRCTN, number 27841311.
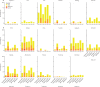
Findings: Between April 19 and May 14, 2021, 1072 participants were enrolled at a median of 9·4 weeks after receipt of a single dose of ChAd (n=540, 47% female) or BNT (n=532, 40% female). In ChAd-primed participants, geometric mean concentration (GMC) 28 days after a boost of SARS-CoV-2 anti-spike IgG in recipients of ChAd/m1273 (20 114 ELISA laboratory units [ELU]/mL [95% CI 18 160 to 22 279]) and ChAd/NVX (5597 ELU/mL [4756 to 6586]) was non-inferior to that of ChAd/ChAd recipients (1971 ELU/mL [1718 to 2262]) with a GMR of 10·2 (one-sided 98·75% CI 8·4 to ∞) for ChAd/m1273 and 2·8 (2·2 to ∞) for ChAd/NVX, compared with ChAd/ChAd. In BNT-primed participants, non-inferiority was shown for BNT/m1273 (GMC 22 978 ELU/mL [95% CI 20 597 to 25 636]) but not for BNT/NVX (8874 ELU/mL [7391 to 10 654]), compared with BNT/BNT (16 929 ELU/mL [15 025 to 19 075]) with a GMR of 1·3 (one-sided 98·75% CI 1·1 to ∞) for BNT/m1273 and 0·5 (0·4 to ∞) for BNT/NVX, compared with BNT/BNT; however, NVX still induced an 18-fold rise in GMC 28 days after vaccination. There were 15 serious adverse events, none considered related to immunisation.
Interpretation: Heterologous second dosing with m1273, but not NVX, increased transient systemic reactogenicity compared with homologous schedules. Multiple vaccines are appropriate to complete primary immunisation following priming with BNT or ChAd, facilitating rapid vaccine deployment globally and supporting recognition of such schedules for vaccine certification.
Funding: UK Vaccine Task Force, Coalition for Epidemic Preparedness Innovations (CEPI), and National Institute for Health Research. NVX vaccine was supplied for use in the trial by Novavax.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM. He receives no personal financial payment for this work. JSN-V-T is seconded to the Department of Health and Social Care, England. AMC and DMF are investigators on studies funded by Pfizer and Unilever. They receive no personal financial payment for this work. AF is a member of the Joint Committee on Vaccination and Immunisation and chair of the WHO European Technical Advisory Group of Experts on Immunisation. He is an investigator or provides consultative advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia. He receives no personal remuneration or benefits for any of this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator or provides consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva. He receives no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, and Valneva. He receives no personal financial payment for this work. CAG acts on behalf of University Hospitals Birmingham NHS Foundation Trust as an investigator on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, CureVac, Moderna, and Valneva. He receives no personal financial payment for this work. VL acts on behalf of University College London Hospitals NHS Foundation Trust as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers including Pfizer, AstraZeneca, and Valneva. He receives no personal financial payment for this work. TL is named as an inventor on a patent application covering the ChAd vaccine and is an occasional consultant to Vaccitech, unrelated to this work. ALG is named as an inventor on a patent covering use of a particular promoter construct that is often used in ChAdOx1-vectored vaccines and is incorporated in the ChAdOx1 nCoV-19 vaccine. ALG benefits from royalty income paid to the University of Oxford from sales of this vaccine by AstraZeneca and its sublicensees under the University's revenue sharing policy. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. All other authors declare no competing interests.
Figures


Comment in
-
Mixing mRNA, adenoviral, and spike-adjuvant vaccines for protection against COVID-19.Lancet. 2022 Jan 1;399(10319):3-5. doi: 10.1016/S0140-6736(21)02757-4. Epub 2021 Dec 6. Lancet. 2022. PMID: 34883051 Free PMC article. No abstract available.
-
Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum.Lancet. 2022 Jan 15;399(10321):234-236. doi: 10.1016/S0140-6736(21)02844-0. Epub 2021 Dec 20. Lancet. 2022. PMID: 34942101 Free PMC article. No abstract available.
References
-
- Our world in data. Statistics and research coronavirus pandemic (COVID-19). https://doi.org/10/2021 2021. https://ourworldindata.org/coronavirus (accessed Nov 30, 2021).
-
- Padma TV. India's COVID-vaccine woes—by the numbers. Nature. 2021;592:500–501. - PubMed
-
- UNICEF COVID-19 Vaccine Market Dashboard. 2021. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
-
- Paterlini M. Covid-19: Sweden, Norway, and Finland suspend use of Moderna vaccine in young people “as a precaution”. BMJ. 2021;375 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

