Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis
- PMID: 34883088
- PMCID: PMC8897230
- DOI: 10.1016/S2213-2600(21)00382-9
Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis
Abstract
Background: Previous latent class analysis of adults with acute respiratory distress syndrome (ARDS) identified two phenotypes, distinguished by the degree of inflammation. We aimed to identify phenotypes in children with ARDS in whom developmental differences might be important, using a latent class analysis approach similar to that used in adults.
Methods: This study was a secondary analysis of data aggregated from the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) clinical trial and the Genetic Variation and Biomarkers in Children with Acute Lung Injury (BALI) ancillary study. We used latent class analysis, which included demographic, clinical, and plasma biomarker variables, to identify paediatric ARDS (PARDS) phenotypes within a cohort of children included in the RESTORE and BALI studies. The association of phenotypes with clinically relevant outcomes and the performance of paediatric data in adult ARDS classification algorithms were also assessed.
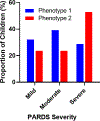
Findings: 304 children with PARDS were included in this secondary analysis. Using latent class analysis, a two-class model was a better fit for the cohort than a one-class model (p<0·001). Latent class analysis identified two classes: class 1 (181 [60%] of 304 patients with PARDS) and class 2 (123 [40%] of 304 patients with PARDS), referred to as phenotype 1 and 2 hereafter. Phenotype 2 was characterised by higher concentrations of inflammatory biomarkers, a higher incidence of vasopressor use, and more frequent diagnosis of sepsis, consistent with the adult hyperinflammatory phenotype. All levels of severity of PARDS were observed across both phenotypes. Children with the hyperinflammatory phenotype (phenotype 2) had worse clinical outcomes than those with the hypoinflammatory phenotype (phenotype 1), with a longer duration of mechanical ventilation (median 10·0 days [IQR 6·3-21·0] for phenotype 2 vs 6·6 days [4·1-10·8] for phenotype 1, p<0·0001), and higher incidence of mortality (17 [13·8%] of 123 patients vs four [2·2%] of 181 patients, p=0·0001). When using adult phenotype classification algorithms in children, the soluble tumour necrosis factor receptor-1 (sTNFr1), vasopressor use, and interleukin (IL)-6 variables gave an area under the curve (AUC) of 0·956, and the sTNFr1, vasopressor use, and IL-8 variables gave an AUC of 0·954, compared with the gold standard of latent class analysis.
Interpretation: Latent class analysis identified two phenotypes in children with ARDS with characteristics similar to those in adults, including worse outcomes among patients with the hyperinflammatory phenotype. PARDS phenotypes should be considered in design and analysis of future clinical trials in children.
Funding: US National Institutes of Health.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MKD, MZ, MAQC, AS, KLD, and HF report grants from the US National Institutes of Health. CSC reports grants from the US National Institutes of Health, Roche/Genentech, Bayer, and Quantum Leap Healthcare Collaborative; and consultancy fees from Quark Pharmaceuticals, Prometic, Gen1e Life Sciences, and Vasomune. MWQ reports consultancy fees from Mitchell Leeds, Tanoury, Nauts, and McKinney and Barbarino. All other authors declare no competing interests.
Figures
References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, and Group ET, Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA, 2016. 315: p. 788–800. - PubMed
-
- Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, Yehya N, Willson D, Kneyber MCJ, Lillie J, Fernandez A, Newth CJL, Jouvet P, Thomas NJ, Pediatric I Acute Respiratory Distress syndrome, I. Epidemiology, I. Pediatric Acute Lung, and N. Sepsis Investigators, Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med, 2018. - PMC - PubMed
-
- Flori HR, Glidden DV, Rutherford GW, and Matthay MA, Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med, 2005. 171: p. 995–1001. - PubMed

