Clofarabine added to intensive treatment in adult patients with newly diagnosed ALL: the HOVON-100 trial
- PMID: 34883506
- PMCID: PMC8864640
- DOI: 10.1182/bloodadvances.2021005624
Clofarabine added to intensive treatment in adult patients with newly diagnosed ALL: the HOVON-100 trial
Abstract
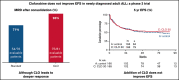
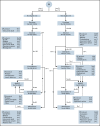
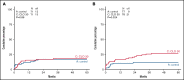
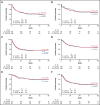
Clofarabine (CLO) is a nucleoside analog with efficacy in relapsed/refractory acute lymphoblastic leukemia (ALL). This randomized phase 3 study aimed to evaluate whether CLO added to induction and whether consolidation would improve outcome in adults with newly diagnosed ALL. Treatment of younger (18-40 years) patients consisted of a pediatric-inspired protocol, and for older patients (41-70 years), a semi-intensive protocol was used. Three hundred and forty patients were randomized. After a median follow-up of 70 months, 5-year event-free survival (EFS) was 50% and 53% for arm A and B (CLO arm). For patients ≤40 years, EFS was 58% vs 65% in arm A vs B, whereas in patients >40 years, EFS was 43% in both arms. Complete remission (CR) rate was 89% in both arms and similar in younger and older patients. Minimal residual disease (MRD) was assessed in 200 patients (60%). Fifty-four of 76 evaluable patients (71%) were MRD- after consolidation 1 in arm A vs 75/81 (93%) in arm B (P = .001). Seventy (42%) patients proceeded to allogeneic hematopoietic stem cell transplantation in both arms. Five-year overall survival (OS) was similar in both arms: 60% vs 61%. Among patients achieving CR, relapse rates were 28% and 24%, and nonrelapse mortality was 16% vs 17% after CR. CLO-treated patients experienced more serious adverse events, more infections, and more often went off protocol. This was most pronounced in older patients. We conclude that, despite a higher rate of MRD negativity, addition of CLO does not improve outcome in adults with ALL, which might be due to increased toxicity. This trial was registered at www.trialregister.nl as #NTR2004.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Dinmohamed AG, Szabó A, van der Mark M, et al. . Improved survival in adult patients with acute lymphoblastic leukemia in the Netherlands: a population-based study on treatment, trial participation and survival. Leukemia. 2016;30(2):310-317. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. ; Eastern Cooperative Oncology Group . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. - PubMed
-
- Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia . Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. - PubMed

