Advanced Ultrasound and Photoacoustic Imaging in Cardiology
- PMID: 34883951
- PMCID: PMC8659598
- DOI: 10.3390/s21237947
Advanced Ultrasound and Photoacoustic Imaging in Cardiology
Abstract
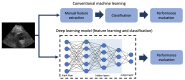
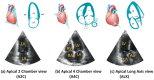
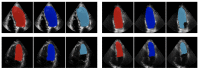
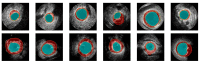
Cardiovascular diseases (CVDs) remain the leading cause of death worldwide. An effective management and treatment of CVDs highly relies on accurate diagnosis of the disease. As the most common imaging technique for clinical diagnosis of the CVDs, US imaging has been intensively explored. Especially with the introduction of deep learning (DL) techniques, US imaging has advanced tremendously in recent years. Photoacoustic imaging (PAI) is one of the most promising new imaging methods in addition to the existing clinical imaging methods. It can characterize different tissue compositions based on optical absorption contrast and thus can assess the functionality of the tissue. This paper reviews some major technological developments in both US (combined with deep learning techniques) and PA imaging in the application of diagnosis of CVDs.
Keywords: cardiovascular diseases; deep learning; photoacoustic imaging; segmentation; ultrasound imaging; vulnerable plaques.
Conflict of interest statement
The authors have no conflict of interest.
Figures

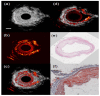
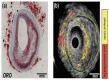
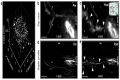
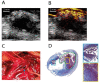


References
-
- World Health Organization . Integrated Management of Cardiovascular Risk. World Health Organization; Geneva, Switzerland: 2002. Cardiovascular Disease Programme; Noncommunicable Disease and Mental Health Cluster.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

