Skin Aging, Cellular Senescence and Natural Polyphenols
- PMID: 34884444
- PMCID: PMC8657738
- DOI: 10.3390/ijms222312641
Skin Aging, Cellular Senescence and Natural Polyphenols
Abstract
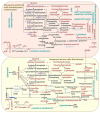
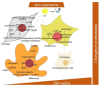
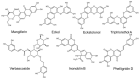
The skin, being the barrier organ of the body, is constitutively exposed to various stimuli impacting its morphology and function. Senescent cells have been found to accumulate with age and may contribute to age-related skin changes and pathologies. Natural polyphenols exert many health benefits, including ameliorative effects on skin aging. By affecting molecular pathways of senescence, polyphenols are able to prevent or delay the senescence formation and, consequently, avoid or ameliorate aging and age-associated pathologies of the skin. This review aims to provide an overview of the current state of knowledge in skin aging and cellular senescence, and to summarize the recent in vitro studies related to the anti-senescent mechanisms of natural polyphenols carried out on keratinocytes, melanocytes and fibroblasts. Aged skin in the context of the COVID-19 pandemic will be also discussed.
Keywords: anti-senescence; natural polyphenols; senescence; skin aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

