Mechanisms, Characteristics, and Treatment of Neuropathic Pain and Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients
- PMID: 34884452
- PMCID: PMC8657961
- DOI: 10.3390/ijms222312648
Mechanisms, Characteristics, and Treatment of Neuropathic Pain and Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients
Abstract
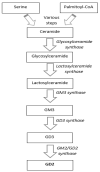
Prognosis of metastatic neuroblastoma is very poor. Its treatment includes induction chemotherapy, surgery, high-dose chemotherapy, radiotherapy, and maintenance with retinoic acid, associated with the anti-GD2 monoclonal antibody (ch14.18) dinutuximab. Immunotherapy determined a significant improvement in survival rate and is also utilized in relapsed and resistant neuroblastoma patients. Five courses of dinutuximab 100 mg/m2 are usually administered as a 10-day continuous infusion or over 5 consecutive days every 5 weeks. Dinutuximab targets the disialoganglioside GD2, which is highly expressed on neuroblastoma cells and minimally present on the surface of normal human neurons, peripheral pain fibers, and skin melanocytes. Anti GD2 antibodies bind to surface GD2 and determine the lysis of neuroblastoma cells induced by immune response via the antibody-dependent cellular cytotoxicity and the complement-dependent cytotoxicity. Dinutuximab has significant side effects, including neuropathic pain, peripheral neuropathy, hypersensitivity reactions, capillary leak syndrome, photophobia, and hypotension. The most important side effect is neuropathic pain, which is triggered by the same antibody-antigen immune response, but generates ectopic activity in axons, which results in hyperalgesia and spontaneous pain. Pain can be severe especially in the first courses of dinutuximab infusion, and requires the administration of gabapentin and continuous morphine infusion. This paper will focus on the incidence, mechanisms, characteristics, and treatment of neuropathic pain and peripheral neuropathy due to dinutuximab administration in neuroblastoma patients.
Keywords: dinutuximab; molecular mechanisms; neuroblastoma; neuropathic pain; pediatric cancer; peripheral neuropathy; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- London W.B., Castleberry R.P., Matthay K.K., Look A., Seeger R.C., Shimada H., Thorner P.S., Brodeur G.M., Maris J.M., Reynolds C.P., et al. Evidence for an Age Cutoff Greater Than 365 Days for Neuroblastoma Risk Group Stratification in the Children’s Oncology Group. J. Clin. Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. - DOI - PubMed
-
- Di Cataldo A., Agodi A., Balaguer J., Garaventa A., Barchitta M., Segura V., Bianchi M., Castel V., Castellano A., Cesaro S., et al. Metastatic Neuroblastoma in Infants: Are Survival Rates Excellent Only within the Stringent Framework of Clinical Trials? Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2017;19:76–83. doi: 10.1007/s12094-016-1505-1. - DOI - PubMed
-
- Matthay K.K., Reynolds C.P., Seeger R.C., Shimada H., Adkins E.S., Haas-Kogan D., Gerbing R.B., London W.B., Villablanca J.G. Long-Term Results for Children with High-Risk Neuroblastoma Treated on a Randomized Trial of Mye-loablative Therapy Followed by 13-Cis-Retinoic Acid: A Children’s Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

