The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited
- PMID: 34884539
- PMCID: PMC8658012
- DOI: 10.3390/ijms222312735
The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited
Abstract
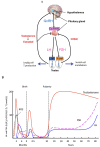
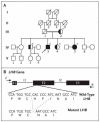
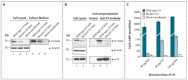
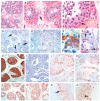
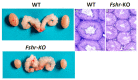
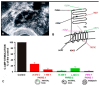
Spermatogenesis and folliculogenesis involve cell-cell interactions and gene expression orchestrated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH). FSH regulates the proliferation and maturation of germ cells independently and in combination with LH. In humans, the requirement for high intratesticular testosterone (T) concentration in spermatogenesis remains both a dogma and an enigma, as it greatly exceeds the requirement for androgen receptor (AR) activation. Several data have challenged this dogma. Here we report our findings on a man with mutant LH beta subunit (LHβ) that markedly reduced T production to 1-2% of normal., but despite this minimal LH stimulation, T production by scarce mature Leydig cells was sufficient to initiate and maintain complete spermatogenesis. Also, in the LH receptor (LHR) knockout (LuRKO) mice, low-dose T supplementation was able to maintain spermatogenesis. In addition, in antiandrogen-treated LuRKO mice, devoid of T action, the transgenic expression of a constitutively activating follicle stimulating hormone receptor (FSHR) mutant was able to rescue spermatogenesis and fertility. Based on rodent models, it is believed that gonadotropin-dependent follicular growth begins at the antral stage, but models of FSHR inactivation in women contradict this claim. The complete loss of FSHR function results in the complete early blockage of folliculogenesis at the primary stage, with a high density of follicles of the prepubertal type. These results should prompt the reassessment of the role of gonadotropins in spermatogenesis, folliculogenesis and therapeutic applications in human hypogonadism and infertility.
Keywords: FSH; LH; Leydig cell; Sertoli cell; azoospermia; folliculogenesis; intratesticular testosterone; knock-out mice; minipuberty; mutation; spermatogenesis; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Huhtaniemi I., Hovatta O., La Marca A., Livera G., Monniaux D., Persani L., Heddar A., Jarzabek K., Laisk-Podar T., Salumets A., et al. Advances in the Molecular Pathophysiology, Genetics, and Treatment of Primary Ovarian Insufficiency. Trends Endocrinol. Metab. 2018;29:400–419. doi: 10.1016/j.tem.2018.03.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

