Human Adenovirus Type 5 Infection Leads to Nuclear Envelope Destabilization and Membrane Permeability Independently of Adenovirus Death Protein
- PMID: 34884837
- PMCID: PMC8657697
- DOI: 10.3390/ijms222313034
Human Adenovirus Type 5 Infection Leads to Nuclear Envelope Destabilization and Membrane Permeability Independently of Adenovirus Death Protein
Abstract
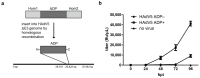
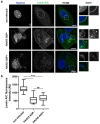
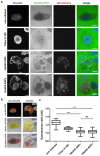
The human adenovirus type 5 (HAdV5) infects epithelial cells of the upper and lower respiratory tract. The virus causes lysis of infected cells and thus enables spread of progeny virions to neighboring cells for the next round of infection. The mechanism of adenovirus virion egress across the nuclear barrier is not known. The human adenovirus death protein (ADP) facilitates the release of virions from infected cells and has been hypothesized to cause membrane damage. Here, we set out to answer whether ADP does indeed increase nuclear membrane damage. We analyzed the nuclear envelope morphology using a combination of fluorescence and state-of-the-art electron microscopy techniques, including serial block-face scanning electron microscopy and electron cryo-tomography of focused ion beam-milled cells. We report multiple destabilization phenotypes of the nuclear envelope in HAdV5 infection. These include reduction of lamin A/C at the nuclear envelope, large-scale membrane invaginations, alterations in double membrane separation distance and small-scale membrane protrusions. Additionally, we measured increased nuclear membrane permeability and detected nuclear envelope lesions under cryoconditions. Unexpectedly, and in contrast to previous hypotheses, ADP did not have an effect on lamin A/C reduction or nuclear permeability.
Keywords: HAdV5; adenovirus death protein; electron cryotomography; membrane damage; nuclear egress; nuclear envelope; serial block-face SEM.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Abou El Hassan M.A.I., van der Meulen-Muileman I., Abbas S., Kruyt F.A.E. Conditionally Replicating Adenoviruses Kill Tumor Cells via a Basic Apoptotic Machinery-Independent Mechanism That Resembles Necrosis-Like Programmed Cell Death. J. Virol. 2004;78:12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

