Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury
- PMID: 34884911
- PMCID: PMC8658203
- DOI: 10.3390/ijms222313106
Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury
Abstract
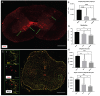
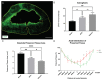
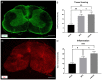
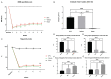
Cervical spinal cord injury (SCI) remains a devastating event without adequate treatment options despite decades of research. In this context, the usefulness of common preclinical SCI models has been criticized. We, therefore, aimed to use a clinically relevant animal model of severe cervical SCI to assess the long-term effects of neural precursor cell (NPC) transplantation on secondary injury processes and functional recovery. To this end, we performed a clip contusion-compression injury at the C6 level in 40 female Wistar rats and a sham surgery in 10 female Wistar rats. NPCs, isolated from the subventricular zone of green fluorescent protein (GFP) expressing transgenic rat embryos, were transplanted ten days after the injury. Functional recovery was assessed weekly, and FluoroGold (FG) retrograde fiber-labeling, as well as manganese-enhanced magnetic resonance imaging (MEMRI), were performed prior to the sacrifice of the animals eight weeks after SCI. After cryosectioning of the spinal cords, immunofluorescence staining was conducted. Results were compared between the treatment groups (NPC, Vehicle, Sham) and statistically analyzed (p < 0.05 was considered significant). Despite the severity of the injury, leading to substantial morbidity and mortality during the experiment, long-term survival of the engrafted NPCs with a predominant differentiation into oligodendrocytes could be observed after eight weeks. While myelination of the injured spinal cord was not significantly improved, NPC treated animals showed a significant increase of intact perilesional motor neurons and preserved spinal tracts compared to untreated Vehicle animals. These findings were associated with enhanced preservation of intact spinal cord tissue. However, reactive astrogliosis and inflammation where not significantly reduced by the NPC-treatment. While differences in the Basso-Beattie-Bresnahan (BBB) score and the Gridwalk test remained insignificant, animals in the NPC group performed significantly better in the more objective CatWalk XT gait analysis, suggesting some beneficial effects of the engrafted NPCs on the functional recovery after severe cervical SCI.
Keywords: NPCs; SCI; functional recovery; neuronal precursor cells; neuroregeneration; spinal cord injury; stem cell therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury.Acta Biomater. 2016 Sep 15;42:77-89. doi: 10.1016/j.actbio.2016.06.016. Epub 2016 Jun 11. Acta Biomater. 2016. PMID: 27296842
-
Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury.Biomaterials. 2014 Mar;35(9):2617-29. doi: 10.1016/j.biomaterials.2013.12.019. Epub 2014 Jan 7. Biomaterials. 2014. PMID: 24406216
-
Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury.Stem Cells Transl Med. 2014 Oct;3(10):1148-59. doi: 10.5966/sctm.2014-0029. Epub 2014 Aug 8. Stem Cells Transl Med. 2014. PMID: 25107585 Free PMC article.
-
Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances.Brain. 2024 Mar 1;147(3):766-793. doi: 10.1093/brain/awad392. Brain. 2024. PMID: 37975820 Review.
-
iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury.Cell Mol Life Sci. 2018 Mar;75(6):989-1000. doi: 10.1007/s00018-017-2676-9. Epub 2017 Oct 9. Cell Mol Life Sci. 2018. PMID: 28993834 Free PMC article. Review.
Cited by
-
Application and prospects of high-throughput screening for in vitro neurogenesis.World J Stem Cells. 2022 Jun 26;14(6):393-419. doi: 10.4252/wjsc.v14.i6.393. World J Stem Cells. 2022. PMID: 35949394 Free PMC article. Review.
-
Spinal cord injury: molecular mechanisms and therapeutic interventions.Signal Transduct Target Ther. 2023 Jun 26;8(1):245. doi: 10.1038/s41392-023-01477-6. Signal Transduct Target Ther. 2023. PMID: 37357239 Free PMC article. Review.
-
The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro.Cells. 2022 Feb 20;11(4):736. doi: 10.3390/cells11040736. Cells. 2022. PMID: 35203385 Free PMC article.
-
Stem cell therapy for central nervous system disorders: Metabolic interactions between transplanted cells and local microenvironments.Neurobiol Dis. 2022 Oct 15;173:105842. doi: 10.1016/j.nbd.2022.105842. Epub 2022 Aug 18. Neurobiol Dis. 2022. PMID: 35988874 Free PMC article. Review.
-
Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model.Front Mol Neurosci. 2023 May 12;16:1128545. doi: 10.3389/fnmol.2023.1128545. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37251648 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

