Targeting MicroRNA-485-3p Blocks Alzheimer's Disease Progression
- PMID: 34884940
- PMCID: PMC8658496
- DOI: 10.3390/ijms222313136
Targeting MicroRNA-485-3p Blocks Alzheimer's Disease Progression
Erratum in
-
Correction: Koh et al. Targeting MicroRNA-485-3p Blocks Alzheimer's Disease Progression. Int. J. Mol. Sci. 2021, 22, 13136.Int J Mol Sci. 2022 Mar 25;23(7):3566. doi: 10.3390/ijms23073566. Int J Mol Sci. 2022. PMID: 35409430 Free PMC article.
Abstract
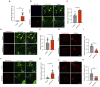
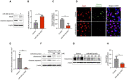
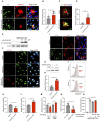
Alzheimer's disease (AD) is a form of dementia characterized by progressive memory decline and cognitive dysfunction. With only one FDA-approved therapy, effective treatment strategies for AD are urgently needed. In this study, we found that microRNA-485-3p (miR-485-3p) was overexpressed in the brain tissues, cerebrospinal fluid, and plasma of patients with AD, and its antisense oligonucleotide (ASO) reduced Aβ plaque accumulation, tau pathology development, neuroinflammation, and cognitive decline in a transgenic mouse model of AD. Mechanistically, miR-485-3p ASO enhanced Aβ clearance via CD36-mediated phagocytosis of Aβ in vitro and in vivo. Furthermore, miR-485-3p ASO administration reduced apoptosis, thereby effectively decreasing truncated tau levels. Moreover, miR-485-3p ASO treatment reduced secretion of proinflammatory cytokines, including IL-1β and TNF-α, and eventually relieved cognitive impairment. Collectively, our findings suggest that miR-485-3p is a useful biomarker of the inflammatory pathophysiology of AD and that miR-485-3p ASO represents a potential therapeutic candidate for managing AD pathology and cognitive decline.
Keywords: Alzheimer’s disease; IL-1β; TNF-α; antisense oligonucleotide; cognitive function; miR-485-3p; microRNA; neuroinflammation; tau; β-amyloid.
Conflict of interest statement
Biorchestra Co. filed a patent on miR-485-3p ASO described in this study, listing J.R. as inventors (serial no. US 16/443,700).
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

