Azobenzene/Tetraethyl Ammonium Photochromic Potassium Channel Blockers: Scope and Limitations for Design of Para-Substituted Derivatives with Specific Absorption Band Maxima and Thermal Isomerization Rate
- PMID: 34884976
- PMCID: PMC8658355
- DOI: 10.3390/ijms222313171
Azobenzene/Tetraethyl Ammonium Photochromic Potassium Channel Blockers: Scope and Limitations for Design of Para-Substituted Derivatives with Specific Absorption Band Maxima and Thermal Isomerization Rate
Abstract
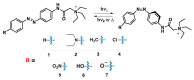
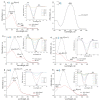
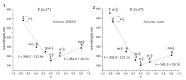
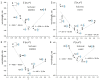
Azobenzene/tetraethyl ammonium photochromic ligands (ATPLs) are photoactive compounds with a large variety of photopharmacological applications such as nociception control or vision restoration. Absorption band maximum and lifetime of the less stable isomer are important characteristics that determine the applicability of ATPLs. Substituents allow to adjust these characteristics in a range limited by the azobenzene/tetraethyl ammonium scaffold. The aim of the current study is to find the scope and limitations for the design of ATPLs with specific spectral and kinetic properties by introducing para substituents with different electronic effects. To perform this task we synthesized ATPLs with various electron acceptor and electron donor functional groups and studied their spectral and kinetic properties using flash photolysis and conventional spectroscopy techniques as well as quantum chemical modeling. As a result, we obtained diagrams that describe correlations between spectral and kinetic properties of ATPLs (absorption maxima of E and Z isomers of ATPLs, the thermal lifetime of their Z form) and both the electronic effect of substituents described by Hammett constants and structural parameters obtained from quantum chemical calculations. The provided results can be used for the design of ATPLs with properties that are optimal for photopharmacological applications.
Keywords: DENAQ; azobenzene; azobenzene thermal isomerization rate; photochromic ion channel blockers; photopharmacology; red-shifting azobenzenes; spectral tuning of azobenzene photoswitches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Red-Shifting Azobenzene Photoswitches for in Vivo Use.Acc Chem Res. 2015 Oct 20;48(10):2662-70. doi: 10.1021/acs.accounts.5b00270. Epub 2015 Sep 28. Acc Chem Res. 2015. PMID: 26415024
-
Kinetic analysis of the thermal isomerisation pathways in an asymmetric double azobenzene switch.Phys Chem Chem Phys. 2012 Apr 7;14(13):4374-82. doi: 10.1039/c2cp23756c. Epub 2012 Feb 22. Phys Chem Chem Phys. 2012. PMID: 22354349
-
Quantum chemical investigation of thermal cis-to-trans isomerization of azobenzene derivatives: substituent effects, solvent effects, and comparison to experimental data.J Phys Chem A. 2009 Jun 18;113(24):6763-73. doi: 10.1021/jp9021344. J Phys Chem A. 2009. PMID: 19453149
-
Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers.Angew Chem Int Ed Engl. 2019 Oct 21;58(43):15421-15428. doi: 10.1002/anie.201905790. Epub 2019 Sep 12. Angew Chem Int Ed Engl. 2019. PMID: 31441199 Review.
-
Photochemical approaches to vision restoration.Vision Res. 2015 Jun;111(Pt B):134-41. doi: 10.1016/j.visres.2015.02.001. Epub 2015 Feb 11. Vision Res. 2015. PMID: 25680758 Free PMC article. Review.
Cited by
-
Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values.Int J Mol Sci. 2023 Jan 26;24(3):2435. doi: 10.3390/ijms24032435. Int J Mol Sci. 2023. PMID: 36768759 Free PMC article. Review.
References
-
- Bregestovski P., Maleeva G. Photopharmacology: A brief review using the control of potassium channels as an example. Neurosci. Behav. Physiol. 2019;49:184–191. doi: 10.1007/s11055-019-00713-3. - DOI
-
- Ryazantsev M.N., Strashkov D.M., Nikolaev D.M., Shtyrov A.A., Panov M.S. Photopharmacological compounds based on azobenzenes and azoheteroarenes: Principles of molecular design, molecular modelling, and synthesis. Russ. Chem. Rev. 2021;90:868. doi: 10.1070/RCR5001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

