Mechanisms and Regulation of Cellular Senescence
- PMID: 34884978
- PMCID: PMC8658264
- DOI: 10.3390/ijms222313173
Mechanisms and Regulation of Cellular Senescence
Abstract
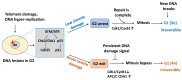
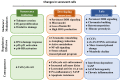
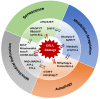
Cellular senescence entails a state of an essentially irreversible proliferative arrest in which cells remain metabolically active and secrete a range of pro-inflammatory and proteolytic factors as part of the senescence-associated secretory phenotype. There are different types of senescent cells, and senescence can be induced in response to many DNA damage signals. Senescent cells accumulate in different tissues and organs where they have distinct physiological and pathological functions. Despite this diversity, all senescent cells must be able to survive in a nondividing state while protecting themselves from positive feedback loops linked to the constant activation of the DNA damage response. This capacity requires changes in core cellular programs. Understanding how different cell types can undergo extensive changes in their transcriptional programs, metabolism, heterochromatin patterns, and cellular structures to induce a common cellular state is crucial to preventing cancer development/progression and to improving health during aging. In this review, we discuss how senescent cells continuously evolve after their initial proliferative arrest and highlight the unifying features that define the senescent state.
Keywords: DNA damage signaling; aging; cell cycle arrest; cellular senescence; epigenetic and chromatin changes; metabolism alteration; mitochondrial dysfunction; senescence-associated secretory phenotype; transcriptome signature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

