Bayesian Networks to Support Decision-Making for Immune-Checkpoint Blockade in Recurrent/Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC)
- PMID: 34884998
- PMCID: PMC8657168
- DOI: 10.3390/cancers13235890
Bayesian Networks to Support Decision-Making for Immune-Checkpoint Blockade in Recurrent/Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC)
Abstract
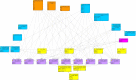
New diagnostic methods and novel therapeutic agents spawn additional and heterogeneous information, leading to an increasingly complex decision-making process for optimal treatment of cancer. A great amount of information is collected in organ-specific multidisciplinary tumor boards (MDTBs). By considering the patient's tumor properties, molecular pathological test results, and comorbidities, the MDTB has to consent an evidence-based treatment decision. Immunotherapies are increasingly important in today's cancer treatment, resulting in detailed information that influences the decision-making process. Clinical decision support systems can facilitate a better understanding via processing of multiple datasets of oncological cases and molecular genetic information, potentially fostering transparency and comprehensibility of available information, eventually leading to an optimum treatment decision for the individual patient. We constructed a digital patient model based on Bayesian networks to combine the relevant patient-specific and molecular data with depended probabilities derived from pertinent studies and clinical guidelines to calculate treatment decisions in head and neck squamous cell carcinoma (HNSCC). In a validation analysis, the model can provide guidance within the growing subject of immunotherapy in HNSCC and, based on its ability to calculate reliable probabilities, facilitates estimation of suitable therapy options. We compared actual treatment decisions of 25 patients with the calculated recommendations of our model and found significant concordance (Cohen's κ = 0.505, p = 0.009) and 84% accuracy.
Keywords: Bayesian network; clinical decision support system (CDSS); head and neck squamous cell carcinoma (HNSCC); immune checkpoint blockade (ICB) targeted therapy; immunotherapy; molecular tumor board; multidisciplinary tumor board.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2023 Dec 29;16(1):175. doi: 10.3390/cancers16010175. Cancers (Basel). 2023. PMID: 38201602 Free PMC article.
-
Head and Neck Cancer Immunotherapy beyond the Checkpoint Blockade.J Dent Res. 2019 Sep;98(10):1073-1080. doi: 10.1177/0022034519864112. Epub 2019 Jul 24. J Dent Res. 2019. PMID: 31340724 Free PMC article. Review.
-
Immunotherapy in Patients with Recurrent and Metastatic Squamous Cell Carcinoma of the Head and Neck.Anticancer Agents Med Chem. 2019;19(3):290-303. doi: 10.2174/1871520618666180910092356. Anticancer Agents Med Chem. 2019. PMID: 30198439 Review.
-
The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer.Phytomedicine. 2021 Nov;92:153758. doi: 10.1016/j.phymed.2021.153758. Epub 2021 Sep 16. Phytomedicine. 2021. PMID: 34592487
-
Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis.Front Immunol. 2021 Apr 7;12:645170. doi: 10.3389/fimmu.2021.645170. eCollection 2021. Front Immunol. 2021. PMID: 33897693 Free PMC article.
Cited by
-
A Treatment Decision Support Model for Laryngeal Cancer Based on Bayesian Networks.Biomedicines. 2023 Jan 1;11(1):110. doi: 10.3390/biomedicines11010110. Biomedicines. 2023. PMID: 36672618 Free PMC article.
-
The data scientist as a mainstay of the tumor board: global implications and opportunities for the global south.Front Digit Health. 2025 Feb 6;7:1535018. doi: 10.3389/fdgth.2025.1535018. eCollection 2025. Front Digit Health. 2025. PMID: 39981102 Free PMC article. No abstract available.
-
Immune Checkpoint Inhibitor Treatment and Ophthalmologist Consultations in Patients with Malignant Melanoma or Lung Cancer-A Nationwide Cohort Study.Cancers (Basel). 2021 Dec 23;14(1):49. doi: 10.3390/cancers14010049. Cancers (Basel). 2021. PMID: 35008211 Free PMC article.
References
-
- Bennardo L., Bennardo F., Giudice A., Passante M., Dastoli S., Morrone P., Provenzano E., Patruno C., Nisticò S. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021;28:2317–2325. doi: 10.3390/curroncol28040213. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

