The Impact of the COVID-19 Pandemic on Oncology Care and Clinical Trials
- PMID: 34885038
- PMCID: PMC8656780
- DOI: 10.3390/cancers13235924
The Impact of the COVID-19 Pandemic on Oncology Care and Clinical Trials
Abstract
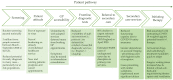
The coronavirus disease 2019 (COVID-19) pandemic has caused considerable global disruption to clinical practice. This article will review the impact that the pandemic has had on oncology clinical trials. It will assess the effect of the COVID-19 situation on the initial presentation and investigation of patients with suspected cancer. It will also review the impact of the pandemic on the subsequent management of cancer patients, and how clinical trial approval, recruitment, and conduct were affected during the pandemic. An intriguing aspect of the pandemic is that clinical trials investigating treatments for COVID-19 and vaccinations against the causative virus, SARS-CoV-2, have been approved and conducted at an unprecedented speed. In light of this, this review will also discuss the potential that this enhanced regulatory environment could have on the running of oncology clinical trials in the future.
Keywords: COVID-19; SARS-Cov-2; cancer screening; clinical trials; oncology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous