Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas
- PMID: 34885050
- PMCID: PMC8656578
- DOI: 10.3390/cancers13235941
Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas
Abstract
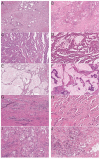
Limited availability of in vivo experimental models for invasive colorectal cancer (CRC) including metastasis and high tumor budding activity is a major problem in colorectal cancer research. In order to compare feline and human intestinal carcinomas, tumors of 49 cats were histologically subtyped, graded and further characterized according to the human WHO classification. Subsequently, feline tumors were compared to a cohort of 1004 human CRC cases. Feline intestinal tumors closely resembled the human phenotype on a histomorphological level. In both species, adenocarcinoma not otherwise specified (ANOS) was the most common WHO subtype. In cats, the second most common subtype of the colon (36.4%), serrated adenocarcinoma (SAC), was overrepresented compared to human CRC (8.7%). Mucinous adenocarcinoma (MAC) was the second most common subtype of the small intestine (12.5%). Intriguingly, feline carcinomas, particularly small intestinal, were generally of high tumor budding (Bd) status (Bd3), which is designated an independent prognostic key factor in human CRC. We also investigated the relevance of feline CTNNB1 exon 2 alterations by Sanger sequencing. In four cases of feline colonic malignancies (3 ANOS, 1 SAC), somatic missense mutations of feline CTNNB1 (p.D32G, p.D32N, p.G34R, and p.S37F) were detected, indicating that mutational alterations of the WNT/β-catenin signaling pathway potentially play an essential role in feline intestinal tumorigenesis comparable to humans and dogs. These results indicate that spontaneous intestinal tumors of cats constitute a useful but so far underutilized model for human CRC. Our study provides a solid foundation for advanced comparative oncology studies and emphasizes the need for further (molecular) characterization of feline intestinal carcinomas.
Keywords: CTNNB1; colorectal cancer; comparative oncology; spontaneous feline intestinal tumors; tumor budding.
Conflict of interest statement
LABOKLIN GmbH & Co. KG offers histopathological service for routine diagnostics. T.G. presented preliminary parts of this study at the 4th Cutting Edge Pathology Congress 2021. W.W. has attended Advisory Boards and served as speaker for Roche, MSD, BMS, AstraZeneca, Pfizer, Merck, Lilly, Boehringer, Novartis, Takeda, Bayer, Amgen, Astellas, Eisai, Illumina, Siemens, Agilent, ADC, GSK, and Molecular Health. W.W. receives research funding from Roche, MSD, BMS and AstraZeneca. N.P. has attended Advisory Boards and served as speaker for Roche, BMS, AstraZeneca, Lilly, Novartis, Bayer, Illumina, and Thermo Fisher Scientific. K.S. is consultant for Roche Pharma AG, member of the advisory board of TRIMT GmbH and has filed a patent on a radiopharmaceutical. All other authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

