Genome Instability in Multiple Myeloma: Facts and Factors
- PMID: 34885058
- PMCID: PMC8656811
- DOI: 10.3390/cancers13235949
Genome Instability in Multiple Myeloma: Facts and Factors
Abstract
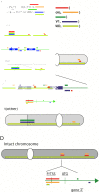
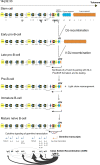
Multiple myeloma (MM) is a malignant neoplasm of terminally differentiated immunoglobulin-producing B lymphocytes called plasma cells. MM is the second most common hematologic malignancy, and it poses a heavy economic and social burden because it remains incurable and confers a profound disability to patients. Despite current progress in MM treatment, the disease invariably recurs, even after the transplantation of autologous hematopoietic stem cells (ASCT). Biological processes leading to a pathological myeloma clone and the mechanisms of further evolution of the disease are far from complete understanding. Genetically, MM is a complex disease that demonstrates a high level of heterogeneity. Myeloma genomes carry numerous genetic changes, including structural genome variations and chromosomal gains and losses, and these changes occur in combinations with point mutations affecting various cellular pathways, including genome maintenance. MM genome instability in its extreme is manifested in mutation kataegis and complex genomic rearrangements: chromothripsis, templated insertions, and chromoplexy. Chemotherapeutic agents used to treat MM add another level of complexity because many of them exacerbate genome instability. Genome abnormalities are driver events and deciphering their mechanisms will help understand the causes of MM and play a pivotal role in developing new therapies.
Keywords: DNA repair; chromothripsis; editing deaminases; genome instability; kataegis; multiple myeloma; translocations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed

