Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients
- PMID: 34885182
- PMCID: PMC8657090
- DOI: 10.3390/cancers13236073
Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients
Abstract
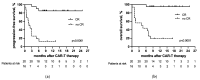
(1) Background: CAR-T-cell therapy is a novel therapeutic option for patients with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL). The parameters that predict a favorable outcome after CAR-T-cell treatment are a matter of ongoing exploration. (2) Methods: We analyzed 36 consecutive patients with r/r DLBCL receiving tisagenlecleucel or axicabtagene ciloleucel at a single academic institution. We hypothesized that lymphoma subtypes (transformed versus de novo DLBCL) are of prognostic importance. We also assessed age, previous treatment, bridging therapy, remission status at the time of CAR-T treatment and at six months, LDH, the occurrence of CRS or ICANS, and CAR-T-DNA ddPCR kinetics for their prognostic impact. (3) Results: CRS was observed in 24 (67%) patients, and ICANS was observed in 14 (39%) patients. CR was achieved in 20 (56%) patients. Achievement of CR within six months after CAR-T was associated with better PFS (p < 0.0001) and OS (p < 0.0001). Remarkably, transformed (=secondary) lymphoma was associated with a better outcome than de novo disease for PFS (p = 0.0093) and OS (p = 0.0209), and the CR rate was 78% versus 33% (p = 0.0176). Mortality in patients with transformed DLBCL was 23% compared with 56% in de novo patients (p = 0.0209). (4) Conclusion: The presence of transformed DLBCL seems to be associated with a more favorable course after CAR-T treatment than that observed in the de novo DLBCL patients.
Keywords: CAR-T-cell therapy; diffuse large B-cell lymphoma (DLBCL); prognosis; relapse; secondary DLBCL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Clinical Trials, U.S. National Library of Medicine Novartis Pharmaceuticals. A Phase II, Single Arm, Multicenter Trial to Determine the Efficacy and Safety of CTL019 in Adult Patients with Relapsed or Refractory Diffuse Large B-cell Lymphoma (DLBCL) [(accessed on 6 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02445248.
-
- European Medicines Agency Yescarta, Axicabtagene Ciloleucel. [(accessed on 6 June 2021)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta.
-
- Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
-
- Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

