Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival
- PMID: 34885185
- PMCID: PMC8656728
- DOI: 10.3390/cancers13236075
Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival
Abstract
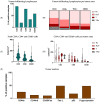
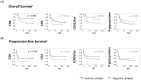
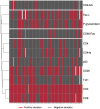
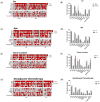
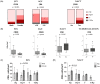
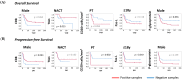
Osteosarcoma (OST) is the most common type of high-grade primary bone tumor, which mainly affects young adults. The current standard of care for OST combines surgical resection with chemotherapy. The clinical outcomes and the current options to treat OST patients are unsatisfactory and novel treatment strategies are needed. The crosstalk between tumor cells and immune cells is essential to the OST microenvironment. Despite the efforts that have been made to address the importance of immune-related factors in OST, there is still a lot to understand. The purpose of the current study was to evaluate the tumor-infiltrating lymphocytes (TIL), the expression of proteins involved in tumor biology, and their impact on the clinical outcome of OST patients. We studied 93 samples of OST patients using immunohistochemistry and histomorphometry. We looked for the infiltration of CD3+, CD4+, CD8+, TIA1+ and CD20+ cells and for the expression of CD44 standard (CD44s) and variant 6 (CD44v6), CD95/Fas, Fas-L, p53 and p-glycoprotein. All the parameters were analyzed for the influence on the occurrence of death and metastasis, plus patient overall survival (OS) and progression-free survival (PFS). The effect of sex, age, tumor location (distal femur or proximal tibia) and the combination with neoadjuvant chemotherapy was also assessed. Our results suggest that the presence of tumor-infiltrating CD4+ cells provides protection to OST patients, and that CD8+ cells have a significant impact on the patient's overall survival (OS) and progression-free survival (PFS), which is more evident in male patients. In addition, a strong association between tumor-infiltrating CD4+ cells and the presence of CD44s expression in tumor samples was observed. Analysis of TIL and tumor markers related to tumor biology could be useful to stratify patients and monitor the response to therapy, as well as to assist with the development of immunotherapy strategies to improve the effects of cytotoxic TIL to eradicate the tumor cells.
Keywords: osteosarcoma; survival; tumor markers; tumor microenvironment; tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
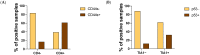
References
-
- Casali P.G., Bielack S., Abecassis N., Aro H.T., Bauer S., Biagini R., Bonvalot S., Boukovinas I., Bovee J.V.M.G., Brennan B., et al. ESMO Guidelines Committee, PaedCan and ERN EURACAN. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv79–iv95. doi: 10.1093/annonc/mdy310. - DOI - PubMed
-
- Geller D.S., Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010;8:705–718. PMID: 21317869. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

