SIOP PNET5 MB Trial: History and Concept of a Molecularly Stratified Clinical Trial of Risk-Adapted Therapies for Standard-Risk Medulloblastoma
- PMID: 34885186
- PMCID: PMC8657236
- DOI: 10.3390/cancers13236077
SIOP PNET5 MB Trial: History and Concept of a Molecularly Stratified Clinical Trial of Risk-Adapted Therapies for Standard-Risk Medulloblastoma
Abstract
Background: SIOP PNET5 MB was initiated in 2014 as the first European trial using clinical, histological, and molecular parameters to stratify treatments for children and adolescents with standard-risk medulloblastoma.
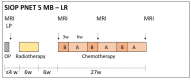
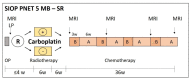
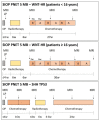
Methods: Stratification by upfront assessment of molecular parameters requires the timely submission of adequate tumour tissue. In the standard-risk phase-III cohort, defined by the absence of high-risk criteria (M0, R0), pathological (non-LCA), and molecular biomarkers (MYCN amplification in SHH-MB or MYC amplification), a randomized intensification by carboplatin concomitant with radiotherapy is investigated. In the LR stratum for localized WNT-activated medulloblastoma and age <16 years, a reduction of craniospinal radiotherapy dose to 18 Gy and a reduced maintenance chemotherapy are investigated. Two additional strata (WNT-HR, SHH-TP53) were implemented during the trial.
Results: SIOP PNET5 MB is actively recruiting. The availability of adequate tumour tissue for upfront real-time biological assessments to assess inclusion criteria has proven feasible.
Conclusion: SIOP PNET5 MB has demonstrated that implementation of biological parameters for stratification is feasible in a prospective multicentre setting, and may improve risk-adapted treatment. Comprehensive research studies may allow assessment of additional parameters, e.g., novel medulloblastoma subtypes, and identification and validation of biomarkers for the further refinement of risk-adapted treatment in the future.
Keywords: CNS; brain tumour; children; medulloblastoma; trial.
Conflict of interest statement
S.R. and F.D. received fees for several advisory board roles. F.D. received fees for his institution for advisory board roles from Bayer, BMS, Roche, Celgene, LOXO Oncology, Servier, Tesaro; travel expenses from Bayer, BMS, Roche; and consultancy roles from Servier. All honoraria contributed to an account at Institut Curie, not to his personal funds. S.R. received fees for advisory board roles from Bayer, Novartis, BMS and Roche; for DMSC from Celgene; and for trial support by German Children’s Cancer Foundation and Riemser Pharma GmbH, Germany. The other authors declare no conflict of interest.
Figures
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Gajjar A., Chintagumpala M., Ashley D., Kellie S., Kun L.E., Merchant T.E., Woo S., Wheeler G., Ahern V., Krasin M.J., et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. - DOI - PubMed
-
- Packer R.J., Gajjar A., Vezina G., Rorke-Adams L., Burger P.C., Robertson P.L., Bayer L., LaFond D., Donahue B.R., Marymont M.H., et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. - DOI - PubMed
Publication types
Grants and funding
- 13457/CRUK_/Cancer Research UK/United Kingdom
- 01GM1909E/BMBF ADDRess
- PHRC-K 2011-250/French Ministry of Health and Institut National du Cancer, and by Société Française de lutte con-tre les Cancers et leucémies de l'Enfant (SFCE)/ Enfants et santé and from Association de Recher-che sur les Tumeurs Cérébrales (ARTC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

