Prognostic Role of PD-L1 Expression in Invasive Breast Cancer: A Systematic Review and Meta-Analysis
- PMID: 34885199
- PMCID: PMC8656531
- DOI: 10.3390/cancers13236090
Prognostic Role of PD-L1 Expression in Invasive Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
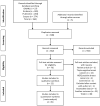
Programmed death ligand 1 (PD-L1) has been investigated in various types of cancer; however, the role of PD-L1 expression in breast cancer remains controversial. We performed a systematic review and meta-analysis to assess the association of PD-L1 expression with clinicopathological variables, overall survival (OS), and disease-free survival (DFS) in invasive breast cancer. A total of 965 articles were included from CINAHL, Embase, PubMed, and Scopus databases. Of these, 22 studies encompassing 6468 cases of invasive breast cancer were included in the systematic review, and 15 articles were included in the meta-analysis. PD-L1 expression was associated with age ≥ 50 years, lymph node status-negative, progesterone receptor-negative, Ki67 ≥ 20%, and human epidermal growth factor receptor 2 (HER2)-negative. PD-L1 positivity was associated with worse OS (hazard ratio, HR, 2.39; 95% confidence interval, CI, 1.26-3.52; p =< 0.000); however, there was no significant improvement in DFS (HR 0.17; 95% CI -0.12-0.46; p =< 0.252). PD-L1 positivity was significantly associated with the clinicopathological characteristics of favorable and unfavorable prognoses. However, the final clinical outcome was associated with lower OS and had no significant association with DFS.
Keywords: PD-L1; breast cancer; immunohistochemistry; prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhang Y., Kang S., Shen J., He J., Jiang L., Wang W., Guo Z., Peng G., Chen G., He J., et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: A meta-analysis. Medicine. 2015;94:e515. doi: 10.1097/MD.0000000000000515. - DOI - PMC - PubMed
-
- Mittendorf E.A., Zhang H., Barrios C.H., Saji S., Jung K.H., Hegg R., Koehler A., Sohn J., Iwata H., Telli M.L., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. - DOI - PubMed
-
- Adams S., Loi S., Toppmeyer D., Cescon D.W., De Laurentiis M., Nanda R., Winer E.P., Mukai H., Tamura K., Armstrong A., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

