Newborn Screening for the Detection of the TP53 R337H Variant and Surveillance for Early Diagnosis of Pediatric Adrenocortical Tumors: Lessons Learned and Way Forward
- PMID: 34885220
- PMCID: PMC8656743
- DOI: 10.3390/cancers13236111
Newborn Screening for the Detection of the TP53 R337H Variant and Surveillance for Early Diagnosis of Pediatric Adrenocortical Tumors: Lessons Learned and Way Forward
Abstract
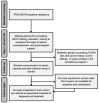
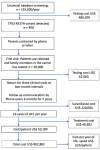
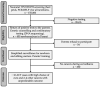
The incidence of pediatric adrenocortical tumors (ACT) is high in southern Brazil due to the founder TP53 R337H variant. Neonatal screening/surveillance (NSS) for this variant resulted in early ACT detection and improved outcomes. The medical records of children with ACT who did not participate in newborn screening (non-NSS) were reviewed (2012-2018). We compared known prognostic factors between the NSS and non-NSS cohorts and estimated surveillance and treatment costs. Of the 16 non-NSS children with ACT carrying the R337H variant, the disease stages I, II, III, and IV were observed in five, five, one, and five children, respectively. The tumor weight ranged from 22 to 608 g. The 11 NSS children with ACT all had disease stage I and were alive. The median tumor weight, age of diagnosis, and interval between symptoms and diagnosis were 21 g, 1.9 years, and two weeks, respectively, for the NSS cohort and 210 g, 5.2 years, and 15 weeks, respectively, for the non-NSS cohort. The estimated surveillance/screening cost per year of life saved is US$623/patient. NSS is critical for improving the outcome of pediatric ACT in this region. Hence, we strongly advocate for the inclusion of R337H in the state-mandated universal screening and surveillance.
Keywords: TP53 R337H; adrenocortical tumor; genetic testing; neonatal screening; surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Custódio G., Parise G.A., Kiesel Filho N., Komechen H., Sabbaga C.C., Rosati R., Grisa L., Parise I.Z., Pianovski M.A., Fiori C.M., et al. Impact of Neonatal Screening and Surveillance for the TP53 R337H Mutation on Early Detection of Childhood Adrenocortical Tumors. J. Clin. Oncol. 2013;31:2619–2626. doi: 10.1200/JCO.2012.46.3711. - DOI - PMC - PubMed
-
- Costa T.E.J., Gerber V.K.Q., Ibañez H.C., Melanda V.S., Parise I.Z.S., Watanabe F.M., Pianovski M.A.D., Fiori C.M.C.M., Fabro A.L.M.R., Silva D.B.D., et al. Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil. Cancers. 2019;11:1804. doi: 10.3390/cancers11111804. - DOI - PMC - PubMed
-
- Ribeiro R.C., Sandrini F., Figueiredo B., Zambetti G.P., Michalkiewicz E., Lafferty A.R., DeLacerda L., Rabin M., Cadwell C., Sampaio G., et al. An Inherited p53 Mutation That Contributes in a Tissue-Specific Manner to Pediatric Adrenal Cortical carcinoma 2001. Proc. Natl. Acad. Sci. USA. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. - DOI - PMC - PubMed
-
- Kerkhofs T.M., Ettaieb M.H., Verhoeven R.H., Kaspers G.J., Tissing W.J., Loeffen J., Van den Heuvel-Eibrink M.M., De Krijger R.R., Haak H.R. Adrenocortical carcinoma in children: First population-based clinicopathological study with long-term follow-up. Oncol. Rep. 2014;32:2836–2844. doi: 10.3892/or.2014.3506. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

