Design, Synthesis and Tumour-Selective Toxicity of Novel 1-[3-{3,5-Bis(benzylidene)-4-oxo-1-piperidino}-3-oxopropyl]-4-piperidone Oximes and Related Quaternary Ammonium Salts
- PMID: 34885719
- PMCID: PMC8659243
- DOI: 10.3390/molecules26237132
Design, Synthesis and Tumour-Selective Toxicity of Novel 1-[3-{3,5-Bis(benzylidene)-4-oxo-1-piperidino}-3-oxopropyl]-4-piperidone Oximes and Related Quaternary Ammonium Salts
Abstract
A novel series of 1-[3-{3,5-bis(benzylidene)-4-oxo-1-piperidino}-3-oxopropyl]-4-piperidone oximes 3a-h and related quaternary ammonium salts 4a-h were prepared as candidate antineoplastic agents. Evaluation against neoplastic Ca9-22, HSC-2 and HSC-4 cells revealed the compounds in series 3 and 4 to be potent cytotoxins with submicromolar CC50 values in virtually all cases. In contrast, the compounds were less cytocidal towards HGF, HPLF and HPC non-malignant cells revealing their tumour-selective toxicity. Quantitative structure-activity relationships revealed that, in general, both cytotoxic potency and selectivity index figures increased as the magnitude of the Hammett sigma values rose. In addition, 3a-h are cytotoxic towards a number of leukemic and colon cancer cells. 4b,c lowered the mitochondrial membrane potential in CEM cells, and 4d induced transient G2/M accumulation in Ca9-22 cells. Five compounds, namely 3c,d and 4c-e, were identified as lead molecules that have drug-like properties.
Keywords: QSAR; conjugated unsaturated ketones; cytotoxins; mitochondrial membrane potential; oximes; quaternary ammonium salts.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



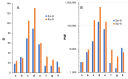

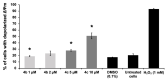
References
-
- Dimmock J., Shyam K., Hamon N., Logan B., Raghavan S., Harwood D., Smith P. Evaluation of Some Mannich Bases Derived from Substituted Acetophenones Against P-388 Lymphocytic Leukemia and on Respiration in Isolated Rat Liver Mitochondria. J. Pharm. Sci. 1983;72:887–894. doi: 10.1002/jps.2600720812. - DOI - PubMed
-
- Mutus B., Wagner J.D., Talpas C.J., Dimmock J.R., Phillips O.A., Reid R. 1-p-Chlorophenyl-4,4-dimethyl-5-diethylamino-1-penten-3-one hydrobromide, a sulfhydryl-specific compound which reacts irreversibly with protein thiols but reversibly with small molecular weight thiols. Anal. Biochem. 1989;177:237–243. doi: 10.1016/0003-2697(89)90045-6. - DOI - PubMed
-
- Addala E., Rafiei H., Das S., Bandy B., Das U., Karki S.S., Dimmock J.R. 3,5-Bis(3-dimethylaminomethyl-4-hydroxybenzylidene)-4-piperidone and related compounds induce glutathione oxidation and mitochondria-mediated cell death in HCT-116 colon cancer cells. Bioorganic Med. Chem. Lett. 2017;27:3669–3673. doi: 10.1016/j.bmcl.2017.07.018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R25 GM069621-16/NIGMS RISE training grant
- 16K11519/Japan Society for the Promotion of Science
- 1SC3GM103713/GM/NIGMS NIH HHS/United States
- 5U54MD007592/National Institute for Minority Health and Health Disparities (NIMHD) the Border Biomedical Research Center at UREP
- RL5GM118969, TL4GM118971 and UL1GM118970/UTEP Building Scholars grants
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

