Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs
- PMID: 34885721
- PMCID: PMC8658949
- DOI: 10.3390/molecules26237146
Towards an Improvement of Anticancer Activity of Benzyl Adenosine Analogs
Abstract
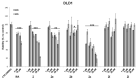
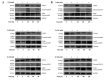
N6-Isopentenyladenosine (i6A) is a naturally occurring modified nucleoside displaying in vitro and in vivo antiproliferative and pro-apoptotic properties. In our previous studies, including an in silico inverse virtual screening, NMR experiments and in vitro enzymatic assays, we demonstrated that i6A targeted farnesyl pyrophosphate synthase (FPPS), a key enzyme involved in the mevalonate (MVA) pathway and prenylation of downstream proteins, which are aberrant in several cancers. Following our interest in the anticancer effects of FPPS inhibition, we developed a panel of i6A derivatives bearing bulky aromatic moieties in the N6 position of adenosine. With the aim of clarifying molecular action of N6-benzyladenosine analogs on the FPPS enzyme inhibition and cellular toxicity and proliferation, herein we report the evaluation of the N6-benzyladenosine derivatives' (compounds 2a-m) effects on cell viability and proliferation on HCT116, DLD-1 (human) and MC38 (murine) colorectal cancer cells (CRC). We found that compounds 2, 2a and 2c showed a persistent antiproliferative effect on human CRC lines and compound 2f exerted a significant effect in impairing the prenylation of RAS and Rap-1A proteins, confirming that the antitumor activity of 2f was related to the ability to inhibit FPPS activity.
Keywords: FPPS; N6-benzyladenosine derivatives; colorectal cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
NMR for screening and a biochemical assay: Identification of new FPPS inhibitors exerting anticancer activity.Bioorg Chem. 2020 May;98:103449. doi: 10.1016/j.bioorg.2019.103449. Epub 2019 Nov 21. Bioorg Chem. 2020. PMID: 32057422
-
The isoprenoid derivative N6 -benzyladenosine CM223 exerts antitumor effects in glioma patient-derived primary cells through the mevalonate pathway.Br J Pharmacol. 2017 Jul;174(14):2287-2301. doi: 10.1111/bph.13824. Epub 2017 Jun 11. Br J Pharmacol. 2017. PMID: 28419419 Free PMC article.
-
N6-isopentenyladenosine arrests tumor cell proliferation by inhibiting farnesyl diphosphate synthase and protein prenylation.FASEB J. 2006 Mar;20(3):412-8. doi: 10.1096/fj.05-4044lsf. FASEB J. 2006. PMID: 16507758
-
Farnesyl pyrophosphate synthase modulators: a patent review (2006 - 2010).Expert Opin Ther Pat. 2011 Sep;21(9):1433-51. doi: 10.1517/13543776.2011.593511. Epub 2011 Jun 25. Expert Opin Ther Pat. 2011. PMID: 21702715 Free PMC article. Review.
-
Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers.Crit Rev Biochem Mol Biol. 2019 Feb;54(1):41-60. doi: 10.1080/10409238.2019.1568964. Epub 2019 Feb 18. Crit Rev Biochem Mol Biol. 2019. PMID: 30773935 Review.
References
-
- Miller C.O., Skoog F., Von Saltza M.H., Strong F. Kinetin, a cell division factor from deoxyribonucleic acid1. J. Am. Chem. Soc. 1955;77:1392. doi: 10.1021/ja01610a105. - DOI
-
- Kersten H. On the biological significance of modified nucleosides in tRNA. Prog. Nucleic Acid Res. Mol. Biol. 1984;31:59–114. - PubMed
-
- Chen C.-m. Cytokinin biosynthesis and interconversion. Physiol. Plant. 1997;101:665–673. doi: 10.1111/j.1399-3054.1997.tb01051.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

