Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies
- PMID: 34885751
- PMCID: PMC8659060
- DOI: 10.3390/molecules26237168
Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies
Abstract
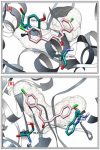
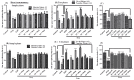
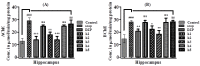
Cognitive decline in dementia is associated with deficiency of the cholinergic system. In this study, five mono-carbonyl curcumin analogs were synthesized, and on the basis of their promising in vitro anticholinesterase activities, they were further investigated for in vivo neuroprotective and memory enhancing effects in scopolamine-induced amnesia using elevated plus maze (EPM) and novel object recognition (NOR) behavioral mice models. The effects of the synthesized compounds on the cholinergic system involvement in the brain hippocampus and their binding mode in the active site of cholinesterases were also determined. Compound h2 (p < 0.001) and h3 (p < 0.001) significantly inhibited the cholinesterases and reversed the effects of scopolamine by significantly reducing TLT (p < 0.001) in EPM, while (p < 0.001) increased the time exploring the novel object. The % discrimination index (DI) was significantly increased (p < 0.001) in the novel object recognition test. The mechanism of cholinesterase inhibition was further validated through molecular docking study using MOE software. The results obtained from the in vitro, in vivo and ex vivo studies showed that the synthesized curcumin analogs exhibited significantly higher memory-enhancing potential, and h3 could be an effective neuroprotective agent. However, more study is suggested to explore its exact mechanism of action.
Keywords: EPM; NORT; amnesia; cholinesterases; hippocampus; molecular docking; mono-carbonyl curcumin analogs; scopolamine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
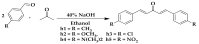


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

