Assessment of Anti-Inflammatory and Antimicrobial Potential of Ethanolic Extract of Woodfordia fruticosa Flowers: GC-MS Analysis
- PMID: 34885782
- PMCID: PMC8659256
- DOI: 10.3390/molecules26237193
Assessment of Anti-Inflammatory and Antimicrobial Potential of Ethanolic Extract of Woodfordia fruticosa Flowers: GC-MS Analysis
Abstract
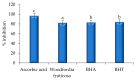
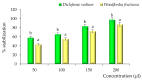
Currently, the potential utilization of natural plant-derived extracts for medicinal and therapeutic purposes has increased remarkably. The current study, therefore, aimed to assess the antimicrobial and anti-inflammatory activity of modified solvent evaporation-assisted ethanolic extract of Woodfordia fruticosa flowers. For viable use of the extract, qualitative analysis of phytochemicals and their identification was carried out by gas chromatography-mass spectroscopy. Analysis revealed that phenolic (65.62 ± 0.05 mg/g), flavonoid (62.82 ± 0.07 mg/g), and ascorbic acid (52.46 ± 0.1 mg/g) components were present in high amounts, while β-carotene (62.92 ± 0.02 µg/mg) and lycopene (60.42 ± 0.8 µg/mg) were present in lower amounts. The antimicrobial proficiency of modified solvent-assisted extract was evaluated against four pathogenic bacterial and one fungal strain, namely Staphylococcusaureus (MTCC 3160), Klebsiellapneumoniae (MTCC 3384), Pseudomonasaeruginosa (MTCC 2295), and Salmonellatyphimurium (MTCC 1254), and Candidaalbicans (MTCC 183), respectively. The zone of inhibition was comparable to antibiotics streptomycin and amphotericin were used as a positive control for pathogenic bacterial and fungal strains. The extract showed significantly higher (p < 0.05) anti-inflammatory activity during the albumin denaturation assay (43.56-86.59%) and HRBC membrane stabilization assay (43.62-87.69%). The extract showed significantly (p < 0.05) higher DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging assay and the obtained results are comparable with BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) with percentage inhibitions of 82.46%, 83.34%, and 84.23%, respectively. Therefore, the obtained results concluded that ethanolic extract of Woodfordia fruticosa flowers could be utilized as a magnificent source of phenols used for the manufacturing of value-added food products.
Keywords: antimicrobial activity; antioxidant activity; chromatography; flower extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
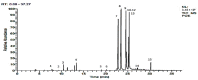
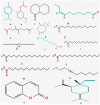


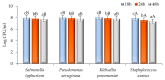
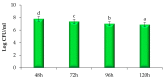


References
-
- Grosser T., Smyth E., FitzGerald G.A. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. Goodman Gilman’s Pharmacol. Basis Ther. 2011;12:959–1004.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

