Expressed Soybean Leghemoglobin: Effect on Escherichia coli at Oxidative and Nitrosative Stress
- PMID: 34885789
- PMCID: PMC8659191
- DOI: 10.3390/molecules26237207
Expressed Soybean Leghemoglobin: Effect on Escherichia coli at Oxidative and Nitrosative Stress
Abstract
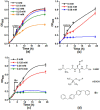
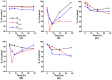
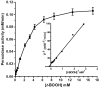
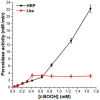
Leghemoglobin (Lb) is an oxygen-binding plant hemoglobin of legume nodules, which participates in the symbiotic nitrogen fixation process. Another way to obtain Lb is its expression in bacteria, yeasts, or other organisms. This is promising for both obtaining Lb in the necessary quantity and scrutinizing it in model systems, e.g., its interaction with reactive oxygen (ROS) and nitrogen (RNS) species. The main goal of the work was to study how Lb expression affected the ability of Escherichia coli cells to tolerate oxidative and nitrosative stress. The bacterium E. coli with the embedded gene of soybean leghemoglobin a contains this protein in an active oxygenated state. The interaction of the expressed Lb with oxidative and nitrosative stress inducers (nitrosoglutathione, tert-butyl hydroperoxide, and benzylviologen) was studied by enzymatic methods and spectrophotometry. Lb formed NO complexes with heme-nitrosylLb or nonheme iron-dinitrosyl iron complexes (DNICs). The formation of Lb-bound DNICs was also detected by low-temperature electron paramagnetic resonance spectroscopy. Lb displayed peroxidase activity and catalyzed the reduction of organic peroxides. Despite this, E. coli-synthesized Lb were more sensitive to stress inducers. This might be due to the energy demand required by the Lb synthesis, as an alien protein consumes bacterial resources and thereby decreases adaptive potential of E. coli.
Keywords: Escherichia coli; dinitrosyl iron complexes; electron paramagnetic resonance; leghemoglobin; nitrosative stress; oxidative stress; protein expression; spectrophotometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Direct detection of radicals in intact soybean nodules: presence of nitric oxide-leghemoglobin complexes.Free Radic Biol Med. 1998 May;24(7-8):1242-9. doi: 10.1016/s0891-5849(97)00440-1. Free Radic Biol Med. 1998. PMID: 9626580
-
Leghemoglobin is nitrated in functional legume nodules in a tyrosine residue within the heme cavity by a nitrite/peroxide-dependent mechanism.Plant J. 2015 Mar;81(5):723-35. doi: 10.1111/tpj.12762. Plant J. 2015. PMID: 25603991 Free PMC article.
-
Excess nitrate induces nodule greening and reduces transcript and protein expression levels of soybean leghaemoglobins.Ann Bot. 2020 Jun 19;126(1):61-72. doi: 10.1093/aob/mcaa002. Ann Bot. 2020. PMID: 32297921 Free PMC article.
-
The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress.Free Radic Biol Med. 2013 Dec;65:1174-1194. doi: 10.1016/j.freeradbiomed.2013.09.001. Epub 2013 Sep 12. Free Radic Biol Med. 2013. PMID: 24036104 Review.
-
Plant hemoglobins: what we know six decades after their discovery.Gene. 2007 Aug 15;398(1-2):78-85. doi: 10.1016/j.gene.2007.01.035. Epub 2007 Apr 25. Gene. 2007. PMID: 17540516 Review.
Cited by
-
Biosynthesis of High-Active Hemoproteins by the Efficient Heme-Supply Pichia Pastoris Chassis.Adv Sci (Weinh). 2023 Oct;10(30):e2302826. doi: 10.1002/advs.202302826. Epub 2023 Aug 30. Adv Sci (Weinh). 2023. PMID: 37649147 Free PMC article.
-
Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin.Int J Mol Sci. 2022 Dec 22;24(1):168. doi: 10.3390/ijms24010168. Int J Mol Sci. 2022. PMID: 36613614 Free PMC article.
-
Production and Analytical Aspects of Natural Pigments to Enhance Alternative Meat Product Color.Foods. 2023 Mar 17;12(6):1281. doi: 10.3390/foods12061281. Foods. 2023. PMID: 36981208 Free PMC article.
-
Metabolic engineering of Bacillus subtilis for the production of active hemoglobins and myoglobins by improving heme supply.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2504795122. doi: 10.1073/pnas.2504795122. Epub 2025 Jul 2. Proc Natl Acad Sci U S A. 2025. PMID: 40601625
-
Nodulation Experiment by Cross-Inoculation of Nitrogen-Fixing Bacteria Isolated from Root Nodules of Several Leguminous Plants.J Microbiol Biotechnol. 2024 Mar 28;34(3):570-579. doi: 10.4014/jmb.2310.10025. Epub 2023 Dec 30. J Microbiol Biotechnol. 2024. PMID: 38213271 Free PMC article.
References
-
- Thony-Meyer L., Preisig O., Zufferey R., Hennecke H. The role of a microaerobically induced cb-type cytochrome oxidase in symbiotic nitrogen fixation. In: Tikhonovich I.A., Provorov N.A., Romanov V.I., Newton W.E., editors. Nitrogen Fixation: Fundamentals and Applications. Springer; Berlin/Heidelberg, Germany: 1995. pp. 383–388.
-
- Singh S., Varma A. Structure, function, and estimation of leghemoglobin. In: Hansen A.P., Choudhary D.K., Agrawal P.K., Varma A., editors. Rhizobium Biology and Biotechnology. Springer; Berlin/Heidelberg, Germany: 2017. pp. 309–330.
-
- Uheda E., Syono K. Effects of leghaemoglobin components on nitrogen fixation and oxygen consumption. Plant Cell Physiol. 1982;23:85–90. doi: 10.1093/oxfordjournals.pcp.a076333. - DOI
-
- Appleby C.A., Bradbury J.H., Morris R.J., Wittenberg B.A., Wittenberg J.B., Wright P.E. Leghemoglobin. Kinetic, nuclear magnetic resonance, and optical studies of pH dependence of oxygen and carbon monoxide binding. J. Biol. Chem. 1983;258:2254–2259. doi: 10.1016/S0021-9258(18)32915-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

