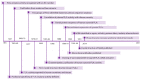
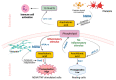
Human Group IIA Phospholipase A2-Three Decades on from Its Discovery
- PMID: 34885848
- PMCID: PMC8658914
- DOI: 10.3390/molecules26237267
Human Group IIA Phospholipase A2-Three Decades on from Its Discovery
Abstract
Phospholipase A2 (PLA2) enzymes were first recognized as an enzyme activity class in 1961. The secreted (sPLA2) enzymes were the first of the five major classes of human PLA2s to be identified and now number nine catalytically-active structurally homologous proteins. The best-studied of these, group IIA sPLA2, has a clear role in the physiological response to infection and minor injury and acts as an amplifier of pathological inflammation. The enzyme has been a target for anti-inflammatory drug development in multiple disorders where chronic inflammation is a driver of pathology since its cloning in 1989. Despite intensive effort, no clinically approved medicines targeting the enzyme activity have yet been developed. This review catalogues the major discoveries in the human group IIA sPLA2 field, focusing on features of enzyme function that may explain this lack of success and discusses future research that may assist in realizing the potential benefit of targeting this enzyme. Functionally-selective inhibitors together with isoform-selective inhibitors are necessary to limit the apparent toxicity of previous drugs. There is also a need to define the relevance of the catalytic function of hGIIA to human inflammatory pathology relative to its recently-discovered catalysis-independent function.
Keywords: cancer; chronic inflammation; drug development; eicosanoid; prostaglandin.
Conflict of interest statement
K.F.S., W.B.C. and P.d.S. are shareholders of Filamon Pty Ltd., which owns intellectual property relating to the use of small peptides and other compounds in the diagnosis and treatment of human disease. None of the employers or funders had any role in the compilation of this review, data collection and analysis, decision to publish or preparation of the manuscript. All other authors declare no potential competing financial, non-financial, professional or personal interests relating to this manuscript.
Figures
References
-
- KEGG Database. [(accessed on 19 October 2021)]. Available online: https://www.genome.jp/dbget-bin/www_bget?ec:3.1.1.4.
-
- OVID Search of Embase Database. [(accessed on 17 October 2021)]. Available online: https://www.embase.com/landing?status=grey.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources