[18F]Nifene PET/CT Imaging in Mice: Improved Methods and Preliminary Studies of α4β2* Nicotinic Acetylcholinergic Receptors in Transgenic A53T Mouse Model of α-Synucleinopathy and Post-Mortem Human Parkinson's Disease
- PMID: 34885943
- PMCID: PMC8659100
- DOI: 10.3390/molecules26237360
[18F]Nifene PET/CT Imaging in Mice: Improved Methods and Preliminary Studies of α4β2* Nicotinic Acetylcholinergic Receptors in Transgenic A53T Mouse Model of α-Synucleinopathy and Post-Mortem Human Parkinson's Disease
Abstract
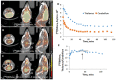
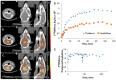
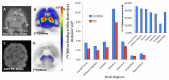
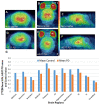
We report [18F]nifene binding to α4β2* nicotinic acetylcholinergic receptors (nAChRs) in Parkinson's disease (PD). The study used transgenic Hualpha-Syn(A53T) PD mouse model of α-synucleinopathy for PET/CT studies in vivo and autoradiography in vitro. Additionally, postmortem human PD brain sections comprising of anterior cingulate were used in vitro to assess translation to human studies. Because the small size of mice brain poses challenges for PET imaging, improved methods for radiosynthesis of [18F]nifene and simplified PET/CT procedures in mice were developed by comparing intravenous (IV) and intraperitoneal (IP) administered [18F]nifene. An optimal PET/CT imaging time of 30-60 min post injection of [18F]nifene was established to provide thalamus to cerebellum ratio of 2.5 (with IV) and 2 (with IP). Transgenic Hualpha-Syn(A53T) mice brain slices exhibited 20-35% decrease while in vivo a 20-30% decrease of [18F]nifene was observed. Lewy bodies and α-synuclein aggregates were confirmed in human PD brain sections which lowered the [18F]nifene binding by more than 50% in anterior cingulate. Thus [18F]nifene offers a valuable tool for PET imaging studies of PD.
Keywords: Hualpha-Syn(A53T); Lewy bodies; PET/CT imaging; Parkinson’s disease; [18F]nifene; transgenic mice; α-synucleinopathy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Reduction in [18F]Nifene Binding, a PET imaging Probe for α4β2* Nicotinic acetylcholinergic receptors in Hippocampus-Subiculum of postmortem human Alzheimer's disease brain.Brain Res. 2025 Jun 15;1857:149600. doi: 10.1016/j.brainres.2025.149600. Epub 2025 Mar 26. Brain Res. 2025. PMID: 40154862 Free PMC article.
-
Disruption of normal brain distribution of [18F]Nifene to α4β2* nicotinic acetylcholinergic receptors in old B6129SF2/J mice and transgenic 3xTg-AD mice model of Alzheimer's disease: In Vivo PET/CT imaging studies.Neuroimage. 2025 Apr 1;309:121092. doi: 10.1016/j.neuroimage.2025.121092. Epub 2025 Feb 18. Neuroimage. 2025. PMID: 39978704 Free PMC article.
-
Abnormal [18 F]NIFENE binding in transgenic 5xFAD mouse model of Alzheimer's disease: In vivo PET/CT imaging studies of α4β2* nicotinic acetylcholinergic receptors and in vitro correlations with Aβ plaques.Synapse. 2023 May;77(3):e22265. doi: 10.1002/syn.22265. Epub 2023 Feb 23. Synapse. 2023. PMID: 36749986 Free PMC article.
-
Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates.Cells. 2025 Jun 16;14(12):907. doi: 10.3390/cells14120907. Cells. 2025. PMID: 40558534 Free PMC article. Review.
-
¹⁸F-FDG PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD010632. doi: 10.1002/14651858.CD010632.pub2. Cochrane Database Syst Rev. 2015. PMID: 25629415 Free PMC article.
Cited by
-
Reduction in [18F]Nifene Binding, a PET imaging Probe for α4β2* Nicotinic acetylcholinergic receptors in Hippocampus-Subiculum of postmortem human Alzheimer's disease brain.Brain Res. 2025 Jun 15;1857:149600. doi: 10.1016/j.brainres.2025.149600. Epub 2025 Mar 26. Brain Res. 2025. PMID: 40154862 Free PMC article.
-
Elevated Monoamine Oxidase-A in Anterior Cingulate of Post-Mortem Human Parkinson's Disease: A Potential Surrogate Biomarker for Lewy Bodies?Cells. 2022 Dec 10;11(24):4000. doi: 10.3390/cells11244000. Cells. 2022. PMID: 36552764 Free PMC article.
-
Development of [124/125I]IAZA as a New Proteinopathy Imaging Agent for Alzheimer's Disease.Molecules. 2023 Jan 15;28(2):865. doi: 10.3390/molecules28020865. Molecules. 2023. PMID: 36677925 Free PMC article.
-
Comparison of Monoamine Oxidase-A, Aβ Plaques, Tau, and Translocator Protein Levels in Postmortem Human Alzheimer's Disease Brain.Int J Mol Sci. 2023 Jun 28;24(13):10808. doi: 10.3390/ijms241310808. Int J Mol Sci. 2023. PMID: 37445985 Free PMC article.
-
[124I]IBETA: A New Aβ Plaque Positron Emission Tomography Imaging Agent for Alzheimer's Disease.Molecules. 2022 Jul 17;27(14):4552. doi: 10.3390/molecules27144552. Molecules. 2022. PMID: 35889425 Free PMC article.
References
-
- Chattopadhyay S., Xue B., Collins D., Pichika R., Bagnera R., Leslie F.M., Christian B.T., Shi B., Narayanan T.K., Potkin S.G., et al. Synthesis and evaluation of nicotine α4β2* receptor radioligand, 5-(3′-fluoropropyl)-3-(2-(S)-pyrrolidinyl)methoxy)pyridine (18F-nifrolidine) in rodents and imaging by PET in non-human primate. J. Nucl. Med. 2005;46:130–140. - PubMed
-
- Pichika R., Easwaramoorthy B., Christian B.T., Shi B., Narayanan T.K., Collins D., Mukherjee J. Nicotine α4β2* receptor imaging agents. Part III. Synthesis and evaluation of 18F-Nifzetidine in rodents and imaging by PET in non-human primate. Nucl. Med. Biol. 2011;38:1183–1192. doi: 10.1016/j.nucmedbio.2011.05.005. - DOI - PMC - PubMed
-
- Pichika R., Kuruvilla S.A., Patel N., Vu K., Sinha S., Easwaramoorthy B., Shi B., Narayanan T.K., Christian B., Mukherjee J. Nicotine α4β2* receptor imaging agents. Part IV. Synthesis and evaluation of [18F]Nifrolene in rodents and non-human primate by PET imaging. Nucl. Med. Biol. 2013;40:117–125. doi: 10.1016/j.nucmedbio.2012.09.009. - DOI - PMC - PubMed
-
- Pichika R., Easwaramoorthy B., Collins D., Christian B.T., Shi B., Narayanan T.K., Potkin S.G., Mukherjee J. Nicotine α4β2* receptor imaging agents. Part II. Synthesis and biological evaluation of 2-[18F]fluoro-3-[2-(S)-pyrrolinyl-3,4-dehydromethoxy]pyridine (18F-Nifene) in rodents and imaging by PET in non-human primate. Nucl. Med. Biol. 2006;33:295–304. doi: 10.1016/j.nucmedbio.2005.12.017. - DOI - PubMed
-
- Mukherjee J., Lao P.J., Betthauser T.J., Samra G.K., Pan M.-L., Patel I.H., Liang C., Metherate R., Christian B.T. Human brain imaging of nicotinic acetylcholine α4β2* receptors using [18F]Nifene: Selectivity, functional activity, toxicity, aging effects, gender effects and extrathalamic pathways. J. Comp. Neurol. 2018;526:80–95. doi: 10.1002/cne.24320. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

