Copper-Catalyzed Ring-Opening Reactions of Alkyl Aziridines with B2pin2: Experimental and Computational Studies
- PMID: 34885983
- PMCID: PMC8659106
- DOI: 10.3390/molecules26237399
Copper-Catalyzed Ring-Opening Reactions of Alkyl Aziridines with B2pin2: Experimental and Computational Studies
Abstract
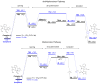
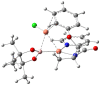
The possibility to form new C-B bonds with aziridines using diboron derivatives continues to be a particularly challenging field in view of the direct preparation of functionalized β-aminoboronates, which are important compounds in drug discovery, being a bioisostere of β-aminoacids. We now report experimental and computational data that allows the individuation of the structural requisites and of reaction conditions necessary to open alkyl aziridines using bis(pinacolate)diboron (B2pin2) in a regioselective nucleophilic addition reaction under copper catalysis.
Keywords: DFT study; aminoboronates; aziridines; copper catalysis; diboron reagents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


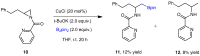



Similar articles
-
Palladium-Catalyzed Regioselective and Stereospecific Ring-Opening Cross-Coupling of Aziridines: Experimental and Computational Studies.Acc Chem Res. 2020 Aug 18;53(8):1686-1702. doi: 10.1021/acs.accounts.0c00395. Epub 2020 Aug 6. Acc Chem Res. 2020. PMID: 32786337
-
Palladium-catalyzed regioselective and stereo-invertive ring-opening borylation of 2-arylaziridines with bis(pinacolato)diboron: experimental and computational studies.Chem Sci. 2016 Sep 1;7(9):6141-6152. doi: 10.1039/c6sc01120a. Epub 2016 Jun 9. Chem Sci. 2016. PMID: 30034753 Free PMC article.
-
CuH-Catalyzed Regioselective Intramolecular Hydroamination for the Synthesis of Alkyl-Substituted Chiral Aziridines.J Am Chem Soc. 2017 Jun 28;139(25):8428-8431. doi: 10.1021/jacs.7b04816. Epub 2017 Jun 15. J Am Chem Soc. 2017. PMID: 28594548 Free PMC article.
-
Nucleophilic ring opening reactions of aziridines.Mol Divers. 2018 May;22(2):447-501. doi: 10.1007/s11030-018-9829-0. Epub 2018 May 4. Mol Divers. 2018. PMID: 29728870 Review.
-
Unsymmetrical Diboron Reagents: Application in Borylation Reactions of Unsaturated Bonds.Molecules. 2019 Apr 4;24(7):1325. doi: 10.3390/molecules24071325. Molecules. 2019. PMID: 30987277 Free PMC article. Review.
Cited by
-
Reactions of Benzylboronate Nucleophiles.Synthesis (Stuttg). 2023 Sep;55(17):2639-2647. doi: 10.1055/a-2072-2754. Epub 2023 May 8. Synthesis (Stuttg). 2023. PMID: 37790600 Free PMC article.
References
-
- Sabir S., Kumar G., Verma V.P., Jat J.L. Aziridine Ring Opening: An Overview of Sustainable Methods. ChemistrySelect. 2018;3:3702–3711. doi: 10.1002/slct.201800170. - DOI
-
- Nonn M., Remete A.M., Fülöp F., Kiss L. Recent advances in the transformations of cycloalkane-fused oxiranes and aziridines. Tetrahedron. 2017;73:5461–5483. doi: 10.1016/j.tet.2017.07.062. - DOI
-
- Pineschi M. Asymmetric Ring-Opening of Epoxides and Aziridines with Carbon Nucleophiles. Eur. J. Org. Chem. 2006;2006:4979–4988. doi: 10.1002/ejoc.200600384. - DOI
-
- Pineschi M. Advances in the Ring Opening of Small-Ring Heterocycles with Organoboron Derivatives. Synlett. 2014;25:1817–1826. doi: 10.1055/s-0033-1339032. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

