Randomized controlled trial of ragweed sublingual immunotherapy tablet in the subpopulation of Canadian children and adolescents with allergic rhinoconjunctivitis
- PMID: 34886880
- PMCID: PMC8656080
- DOI: 10.1186/s13223-021-00626-2
Randomized controlled trial of ragweed sublingual immunotherapy tablet in the subpopulation of Canadian children and adolescents with allergic rhinoconjunctivitis
Abstract
Background: Post hoc analyses of randomized placebo-controlled trials have demonstrated efficacy and tolerability of the ragweed sublingual immunotherapy (SLIT)-tablet in Canadian adults with ragweed pollen-induced allergic rhinitis/conjunctivitis (AR/C). This post hoc analysis evaluated the efficacy and tolerability of the ragweed SLIT-tablet in the subpopulation of Canadian children and adolescents with AR/C in a previously described randomized, double-blind, placebo-controlled trial.
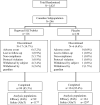
Methods: The trial (NCT02478398) was conducted in North American and European children/adolescents ages 5-17 years with ragweed pollen-induced AR/C with or without asthma (FEV1 ≥ 80% predicted). Participants were randomized to daily ragweed SLIT-tablet (12 Amb a 1-U) or placebo for up to 28 weeks. The primary endpoint was the average total combined score (TCS; sum of rhinoconjunctivitis daily symptom score [DSS] and daily medication score [DMS]) during peak ragweed pollen season (RPS). Key secondary endpoints were TCS during the entire RPS, and DSS and DMS during peak RPS. Post hoc analyses of the primary and key secondary endpoints were conducted in the subpopulation of Canadian participants.
Results: Of the 1025 randomized participants, 246 (SLIT-tablet, n = 116; placebo, n = 130) were in the Canadian subpopulation. In the total study population, relative TCS (95% CI) improvement with ragweed SLIT-tablet versus placebo was - 38.3% (- 46.0%, - 29.7%; least square [LS] mean difference, - 2.73; P < 0.001) during peak RPS. In the Canadian subpopulation, relative TCS improvements with ragweed SLIT-tablet versus placebo were - 40.8% (- 54.5%, - 20.2%; LS mean difference, - 1.59; P = 0.001) during peak RPS and - 36.6% (- 50.2%, - 16.5%; LS mean difference, - 1.36; P = 0.002) during the entire RPS. DSS and DMS during peak RPS in the Canadian subpopulation improved with SLIT-tablet versus placebo by - 30.6% (- 45.2%, - 7.7%; LS mean difference, - 0.94; P = 0.010) and - 77.2% (- 97.5%, - 44.2%; LS mean difference, - 0.66; P = 0.003), respectively. No events of anaphylaxis, airway compromise, intramuscular epinephrine administration, eosinophilic esophagitis, or severe treatment-related systemic allergic reactions were reported in the overall population or Canadian subpopulation.
Conclusion: Efficacy and safety of the ragweed SLIT-tablet in Canadian children/adolescents with ragweed pollen-induced AR/C was consistent with the total study population. The ragweed SLIT-tablet resulted in clinically meaningful improvement in symptoms, decreased symptom-relieving medication use, and was well tolerated in Canadian children/adolescents.
Trial registration: clinicaltrials.gov, NCT02478398. Registered June 23, 2015, https://clinicaltrials.gov/ct2/show/NCT02478398?term=NCT02478398&draw=2&rank=1.
Keywords: Adolescents; Allergic rhinoconjunctivitis; Children; Ragweed; Sublingual immunotherapy.
© 2021. The Author(s).
Conflict of interest statement
D.I. Bernstein has received grant support from Aimmune, Allergy Therapeutics, ALK, Amgen, AstraZeneca, Avillion, Biocryst, Boehringer Ingelheim, Cipla, Genentech, GlaxoSmithKline, Gossamer, Leo, Lupin, Menlo, Merck, Mylan, Novartis, Novum, Pearl, Regeneron, Shire, and TEVA, served as an advisor for ALK America, Gerson-Lehman, GlaxoSmithKline, and Guidepoint Global, and been a speaker for ALK and GlaxoSmithKline. A.K. Ellis has participated in advisory boards for Abbvie, ALK-Abelló, AstraZeneca, Aralez, Bausch Health, Circassia Ltd, GlaxoSmithKline, LEO Pharma, Johnson & Johnson, Merck, Mylan, Novartis, Pediapharm and Pfizer, has been a speaker for ALK, Aralez, AstraZeneca, Bausch Health, Boehringer-Ingelheim, CACME, Meda, Medexus, Mylan, Merck, Novartis, Pediapharm, Pfizer, The ACADEMY, and Takeda. Her institution has received research grants from AstraZeneca, Bayer, LLC, Circassia Ltd, Green Cross Pharmaceuticals, GlaxoSmithKline, Sun Pharma, Merck, Novartis, Pfizer, Regeneron and Sanofi. She has also served as an independent consultant to Allergy Therapeutics, Bayer, LLC, Ora Inc., and Regeneron in the past. R. Gagnon has served as a speaker and/or advisor for ALK-Abello, AstraZeneca, Aralez, CSL Behring, GlaxoSmithKline, Merck, Novartis, Pediapharm, Pfizer, Sanofi, and Shire, and served as an investigator for AstraZeneca, Biocryst, DBV, GlaxoSmithKline, Green Cross Pharmaceuticals, Merck, Novartis, Regeneron, Shire, Stallergenes, and Sanofi Genzyme/Regeneron. H. Nolte is an employee of ALK.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

