Provider response and follow-up to parental declination of HPV vaccination
- PMID: 34887133
- PMCID: PMC8755625
- DOI: 10.1016/j.vaccine.2021.11.055
Provider response and follow-up to parental declination of HPV vaccination
Abstract
Objective: Parents often decline HPV vaccination, but little is known about how healthcare providers should promote vaccination at a later visit for secondary acceptance. We examined the associations of two factors, providers' response to declination during the visit and follow-up after the visit, with secondary acceptance.
Methods: We conducted a cross-sectional survey of US parents whose 9- to 17-year-old child had not yet completed the HPV vaccination series. Parents who declined HPV vaccination during an initial discussion with a provider (n = 447) reported whether their provider engaged in any active response during the visit (e.g., giving information, trying to change their mind) or any follow-up after the visit (e.g., scheduling another visit). We conducted multivariable logistic regression to determine whether an active response or follow-up was associated with secondary acceptance of HPV vaccination.
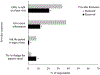
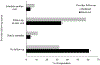
Results: Only about one-third of parents reported an active response during the visit (35%) or follow-up after the visit (39%) following HPV vaccination declination. Parents had higher odds of secondary acceptance of HPV vaccine if they received any provider follow-up after the visit (43% vs. 20%, aOR:3.19; 95% CI:2.00:5.07). Receipt of an active provider response was not associated with secondary acceptance. More parents thought a provider should actively respond and follow-up (61% and 68% respectively), compared with those who received such a response (both p < .01).
Conclusions: Providers' follow-up after the visit may be important for promoting secondary acceptance of HPV vaccination. Parents who decline HPV vaccination often prefer to receive an active response or follow-up from a provider.
Keywords: Adolescent health; Human papillomavirus infections/prevention & control; Human papillomavirus vaccine; Patient-provider communication.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Although not in the past three years, we do wish to share that Dr. Brewer has received vaccine-related grants and honoraria from or served on paid advisory boards for Merck Sharp & Dohme Corp, Pfizer and GlaxoSmithKline.
Figures
References
-
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. The New England journal of medicine. 2015;372(8):711–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources