Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry
- PMID: 34887335
- PMCID: PMC9364353
- DOI: 10.2967/jnumed.121.262713
Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry
Abstract
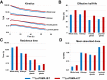
The objective of this study was to determine the safety, kinetics, and dosimetry of the 177Lu-labeled prostate-specific membrane antigen (PSMA) small molecules 177Lu-PSMA I&T and 177Lu-PSMA-617 in a large cohort of patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing PSMA radioligand therapy (PRLT). Methods: In total, 138 patients (mean age, 70 ± 9 y; age range, 46-90 y) with progressive mCRPC and PSMA expression verified by 68Ga-PSMA-11 PET/CT underwent PRLT. Fifty-one patients received 6.1 ± 1.0 GBq (range, 3.4-7.6 GBq) of 177Lu-PSMA I&T, and 87 patients received 6.5 ± 1.1 GBq (range, 3.5-9.0 GBq) of 177Lu-PSMA-617. Dosimetry was performed on all patients using an identical protocol. The mean absorbed doses were estimated with OLINDA software (MIRD Scheme). Treatment-related adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 5.0, of the National Cancer Institute. Results: The whole-body half-lives were shorter for 177Lu-PSMA I&T (35 h) than for 177Lu-PSMA-617 (42 h). The mean whole-body dose of 177Lu-PSMA-617 was higher than that of 177Lu-PSMA I&T (0.04 vs. 0.03 Gy/GBq, P < 0.00001). Despite the longer half-life of 177Lu-PSMA-617, the renal dose was lower for 177Lu-PSMA-617 than for 177Lu-PSMA I&T (0.77 vs. 0.92 Gy/GBq, P = 0.0015). Both PSMA small molecules demonstrated a comparable dose to the parotid glands (0.5 Gy/GBq, P = 0.27). Among all normal organs, the lacrimal glands exhibited the highest mean absorbed doses, 5.1 and 3.7 Gy/GBq, for 177Lu-PSMA-617 and 177Lu-PSMA I&T, respectively. All tumor metastases exhibited a higher initial uptake when using 177Lu-PSMA I&T than when using 177Lu-PSMA-617, as well as a shorter tumor half-life (P < 0.00001). The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617 (5.8 vs. 5.9 Gy/GBq, P = 0.96). All patients tolerated the therapy without any acute adverse effects. After 177Lu-PSMA-617 and 177Lu-PSMA I&T, there was a small, statistically significant reduction in hemoglobin, leukocyte counts, and platelet counts that did not need any clinical intervention. No nephrotoxicity was observed after either 177Lu-PSMA I&T or 177Lu-PSMA-617 PRLT. Conclusion: Both 177Lu-PSMA I&T and 177Lu-PSMA-617 PRLT demonstrated favorable safety in mCRPC patients. The highest absorbed doses among healthy organs were in the lacrimal and parotid glands-not, however, resulting in any significant clinical sequel. 177Lu-PSMA-617 demonstrated a higher absorbed dose to the whole-body and lacrimal glands but a lower renal dose than did 177Lu-PSMA I&T. The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617. There was a large interpatient variability in the dosimetry parameters. Therefore, individual patient-based dosimetry seems favorable for personalized PRLT.
Keywords: 177Lu; 177Lu-PSMA I&T; 177Lu-PSMA-617; PSMA radioligand therapy; dosimetry; prostate-specific membrane antigen; theranostics.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Halabi S, Vogelzang NJ, Kornblith AB, et al. . Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–2549. - PubMed
-
- Hofman MS, Violet J, Hicks RJ, et al. . [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. - PubMed
-
- Hofman MS, Emmett L, Sandhu S, et al. . [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
