HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3β via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas
- PMID: 34887392
- PMCID: PMC8660812
- DOI: 10.1038/s41419-021-04440-x
HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3β via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas
Erratum in
-
Correction to: HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3β via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas.Cell Death Dis. 2022 Feb 21;13(2):164. doi: 10.1038/s41419-022-04547-9. Cell Death Dis. 2022. PMID: 35190524 Free PMC article. No abstract available.
Abstract
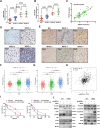
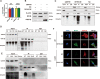
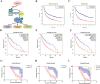
Myosin heavy chain 9 (MYH9) plays an essential role in human diseases, including multiple cancers; however, little is known about its role in gliomas. In the present study, we revealed that HMGA1 and MYH9 were upregulated in gliomas and their expression correlated with WHO grade, and HMGA1 promoted the acquisition of malignant phenotypes and chemoresistance of glioma cells by regulating the expression of MYH9 through c-Jun-mediated transcription. Moreover, MYH9 interacted with GSK-3β to inhibit the expression of GSK-3β protein by promoting its ubiquitination; the downregulation of GSK-3β subsequently promoted the nuclear translocation of β-catenin, enhancing growth, invasion, migration, and temozolomide resistance in glioma cells. Expression levels of HMGA1 and MYH9 were significantly correlated with patient survival and should be considered as independent prognostic factors. Our findings provide new insights into the role of HMGA1 and MYH9 in gliomagenesis and suggest the potential application of HMGA1 and MYH9 in cancer therapy in the future.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Maher EA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–33. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. - PubMed
-
- Emdad L, Dent P, Sarkar D, Fisher PB. Future approaches for the therapy of malignant glioma: targeting genes mediating invasion. Future Oncol. 2012;8:343–6. - PubMed
-
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–22. - PubMed
-
- Zhao C, Li Y, Zhang W, Zhao D, Ma L, Ma P, et al. IL17 induces NSCLC A549 cell proliferation via the upregulation of HMGA1, resulting in an increased cyclin D1 expression. Int J Oncol. 2018;52:1579–1592. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

